Question
Question: 3 Faradays of electricity is passed through solution of CuSO₄. The number of moles of copper deposit...
3 Faradays of electricity is passed through solution of CuSO₄. The number of moles of copper deposited on the cathode will be.
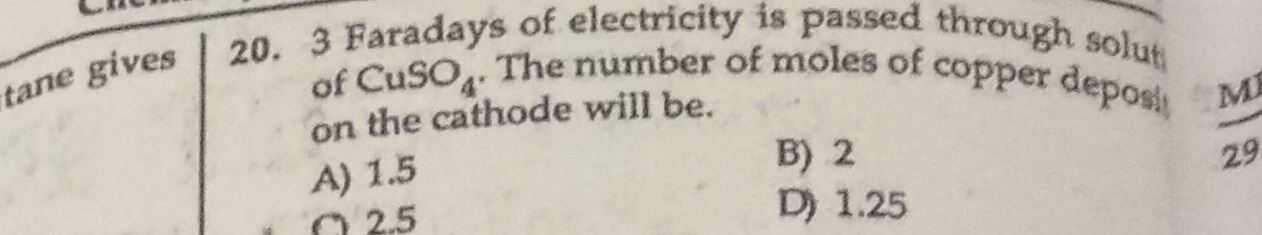
A
1.5
B
2
C
2.5
D
1.25
Answer
1.5
Explanation
Solution
The half-reaction is:
Cu2++2e−⟶CuHere, 2 moles of electrons (2 Faradays) deposit 1 mole of copper. Therefore, with 3 Faradays:
Moles of Cu=23=1.5moles