Question
Question: Which of the following reactions is NOT exothermic?...
Which of the following reactions is NOT exothermic?
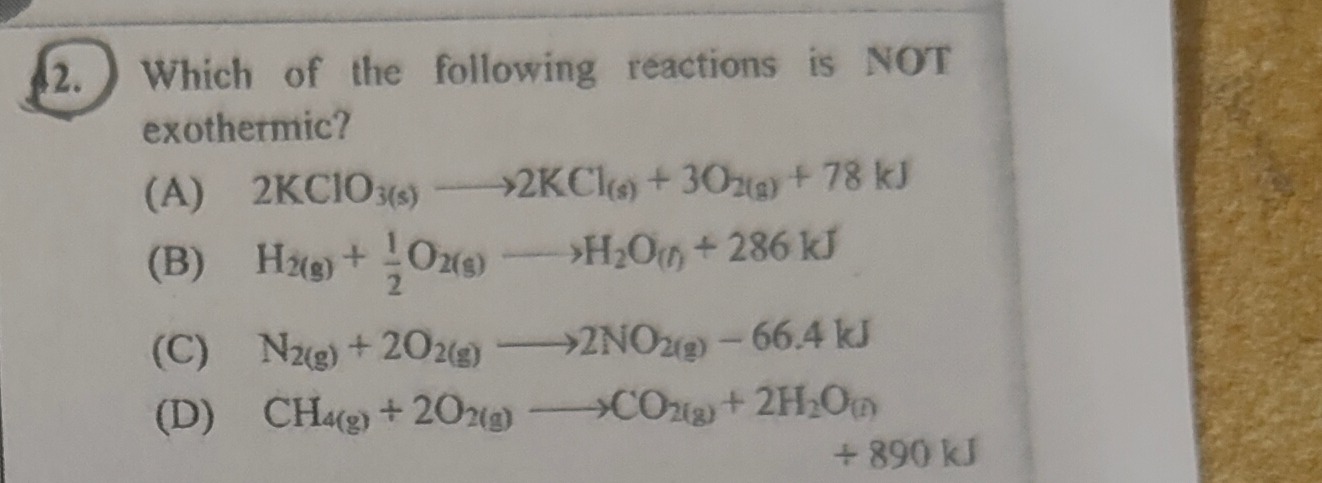
A
2KClO3(s)⟶2KCl(s)+3O2(g)+78 kJ
B
H2(g)+21O2(g)⟶H2O(l)+286 kJ
C
N2(g)+2O2(g)⟶2NO2(g)−66.4 kJ
D
CH4(g)+2O2(g)⟶CO2(g)+2H2O(l)+890 kJ
Answer
Option (C)
Explanation
Solution
For an exothermic reaction, energy is released (indicated by a positive value next to "kJ").
- Reactions (A), (B), and (D) release energy (+78 kJ, +286 kJ, and +890 kJ, respectively).
- Reaction (C) absorbs energy (-66.4 kJ), indicating an endothermic reaction.
Therefore, the reaction that is NOT exothermic is Option (C).
