Question
Question: Which of the following alkyl halide undergo rearrangement in $S_N1$ reaction? a. b. c. $CH_3- \un...
Which of the following alkyl halide undergo rearrangement in SN1 reaction?
a.
b.
c. CH3−CH3∣CH3C−ICH∣−CH3
d. All of these
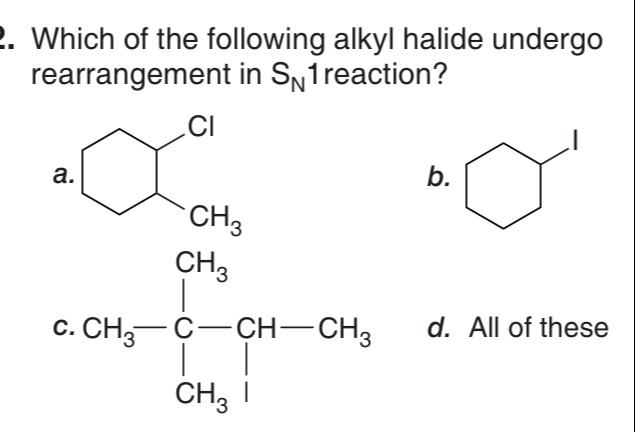
a.
b.
c. CH3−CH3∣CH3C−ICH∣−CH3
d. All of these
d. All of these
Solution
The SN1 reaction proceeds through the formation of a carbocation intermediate. Rearrangement of the carbocation occurs if a more stable carbocation can be formed from the initially formed carbocation through a 1,2-hydride shift or 1,2-alkyl shift. The stability of carbocations increases in the order: methyl < primary < secondary < tertiary < resonance-stabilized.
Let's analyze each given alkyl halide:
a. 1-chloro-2-methylcyclohexane. The chlorine is attached to a secondary carbon (C1). Upon ionization, a secondary carbocation is formed at C1. The adjacent carbon (C2) is bonded to a methyl group and a hydrogen atom. A 1,2-hydride shift from C2 to C1 moves the positive charge to C2. The carbocation at C2 is bonded to C1, C3, and the methyl group, making it a tertiary carbocation. Since a tertiary carbocation is more stable than a secondary carbocation, rearrangement occurs.
b. Iodocyclohexane. The iodine is attached to a secondary carbon (C1). Upon ionization, a secondary carbocation is formed at C1. The adjacent carbons (C2 and C6) are also secondary carbons with hydrogen atoms. A 1,2-hydride shift from C2 to C1 forms a secondary carbocation at C2. Similarly, a 1,2-hydride shift from C6 to C1 forms a secondary carbocation at C6. Neither of these shifts leads to a more stable carbocation. Therefore, rearrangement does not occur in (b) under typical conditions.
c. 2-iodo-3,3-dimethylbutane. The iodine is attached to a secondary carbon (C2). Upon ionization, a secondary carbocation is formed at C2. The adjacent carbon (C3) is a tertiary carbon bonded to three methyl groups. A 1,2-methyl shift from C3 to C2 moves the positive charge to C3. The carbocation at C3 is bonded to C2 and three methyl groups, making it a tertiary carbocation. Since a tertiary carbocation is more stable than a secondary carbocation, rearrangement occurs.
Based on the analysis, alkyl halides (a) and (c) undergo rearrangement in SN1 reactions.
