Question
Question: The correct order of stability is/are...
The correct order of stability is/are
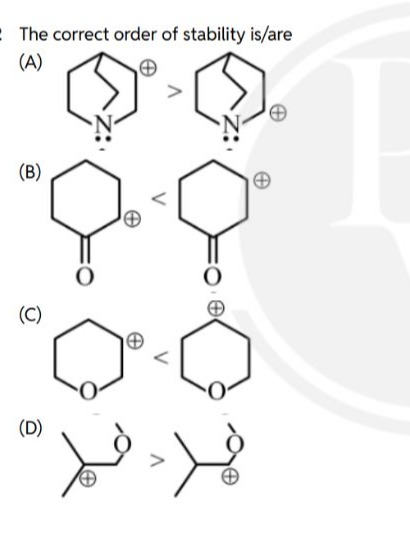
<
<
A, B, D
Solution
To determine the correct order of stability for the given carbocations, we will analyze each option based on principles of carbocation stability, including resonance, inductive effects, hyperconjugation, and ring strain.
General Principles of Carbocation Stability:
-
Resonance Stabilization: Carbocations adjacent to atoms with lone pairs (like N, O, S) are highly stabilized because the lone pair can be donated to the empty p-orbital of the carbocation, forming a double bond and completing the octet of the carbon. This effect (e.g.,
C+-X:↔C=X+) is very strong. -
Hyperconjugation: Alkyl groups stabilize carbocations by donating electron density through hyperconjugation. More alkyl groups lead to greater stability (tertiary > secondary > primary).
-
Inductive Effects: Electron-donating groups (+I effect) stabilize carbocations, while electron-withdrawing groups (-I effect) destabilize them.
-
Ring Strain: In cyclic and bicyclic systems, the geometry required for optimal orbital overlap (e.g., for resonance) can be hindered by ring strain, reducing stability.
Let's evaluate each option:
Option (A):
-
Structure A1: A secondary carbocation on a carbon atom alpha to the nitrogen in a 1-azabicyclo[2.2.2]octane derivative. The nitrogen atom has a lone pair. This is an α-amino carbocation.
-
Structure A2: A secondary carbocation on a carbon atom alpha to the nitrogen in a 1-azabicyclo[2.2.1]heptane derivative. This is also an α-amino carbocation.
Both A1 and A2 are highly stabilized by resonance with the nitrogen lone pair (C+-N: ↔ C=N+). The difference lies in the ring strain. The 1-azabicyclo[2.2.1]heptane system (A2) contains a more strained 5-membered ring compared to the 1-azabicyclo[2.2.2]octane system (A1) which has a 6-membered ring. The increased strain in A2 might hinder the ability of the carbocation and nitrogen to achieve the planar geometry required for optimal p-orbital overlap in the resonance structure. Therefore, the less strained system (A1) allows for better resonance stabilization.
Conclusion for (A): A1 > A2. This order is correct.
Option (B):
-
Structure B1: A carbocation on the carbon atom alpha to the carbonyl group in cyclohexanone.
-
Structure B2: A carbocation on the carbon atom beta to the carbonyl group in cyclohexanone.
The carbonyl group (C=O) is strongly electron-withdrawing due to both inductive (-I) and resonance (-M) effects.
-
In B1 (α-carbonyl carbocation), the positive charge is adjacent to the electron-withdrawing carbonyl group. This significantly destabilizes the carbocation. Any resonance form (e.g.,
C=C-O+) would place a positive charge on the electronegative oxygen, which is unfavorable. -
In B2 (β-carbonyl carbocation), the positive charge is further away. The inductive destabilization is much weaker at the beta position, and there is no direct resonance interaction.
Therefore, B2 is more stable than B1.
Conclusion for (B): B1 < B2. This order is correct.
Option (C):
-
Structure C1: A carbocation on the carbon atom alpha to the oxygen in tetrahydropyran (a cyclic ether).
-
Structure C2: A carbocation on the carbon atom beta to the oxygen in tetrahydropyran.
-
In C1 (α-ether carbocation), the positive charge is adjacent to the oxygen atom, which has lone pairs. The oxygen can donate its lone pair to stabilize the carbocation through resonance (
C+-O:↔C=O+). This effect is very powerful as it completes the octet of the carbon atom. -
In C2 (β-ether carbocation), the positive charge is at the beta position. The oxygen atom exerts an electron-withdrawing inductive effect (-I), which destabilizes the carbocation. There is no resonance stabilization.
Therefore, C1 is significantly more stable than C2.
Conclusion for (C): C1 > C2. The given order C1 < C2 is incorrect.
Option (D):
-
Structure D1: A secondary carbocation alpha to an ether oxygen:
(CH3)2CH-CH+-OCH3. -
Structure D2: A primary carbocation beta to an ether oxygen:
CH2+-CH2-O-CH(CH3)2. -
D1 is an α-alkoxy carbocation. It is highly stabilized by resonance with the oxygen lone pair (
C+-O:↔C=O+). This stabilization is so strong that α-alkoxy carbocations are often more stable than tertiary alkyl carbocations. -
D2 is a primary carbocation, which is inherently unstable. Additionally, the beta-oxygen exerts an electron-withdrawing inductive effect (-I), further destabilizing it.
Therefore, D1 is significantly more stable than D2.
Conclusion for (D): D1 > D2. This order is correct.
Based on the analysis, options (A), (B), and (D) present correct orders of stability.
