Question
Question: Starting at temperature 300 K, one mole of an ideal diatomic gas ($\gamma$ = 1.4) is first compresse...
Starting at temperature 300 K, one mole of an ideal diatomic gas (γ = 1.4) is first compressed adiabatically from volume V1 to V2=16V1. It is then allowed to expand isobarically to volume 2V2. If all the processes are the quasi-static, then the final temperature of the gas (in K) is (to the nearest integer) ____.
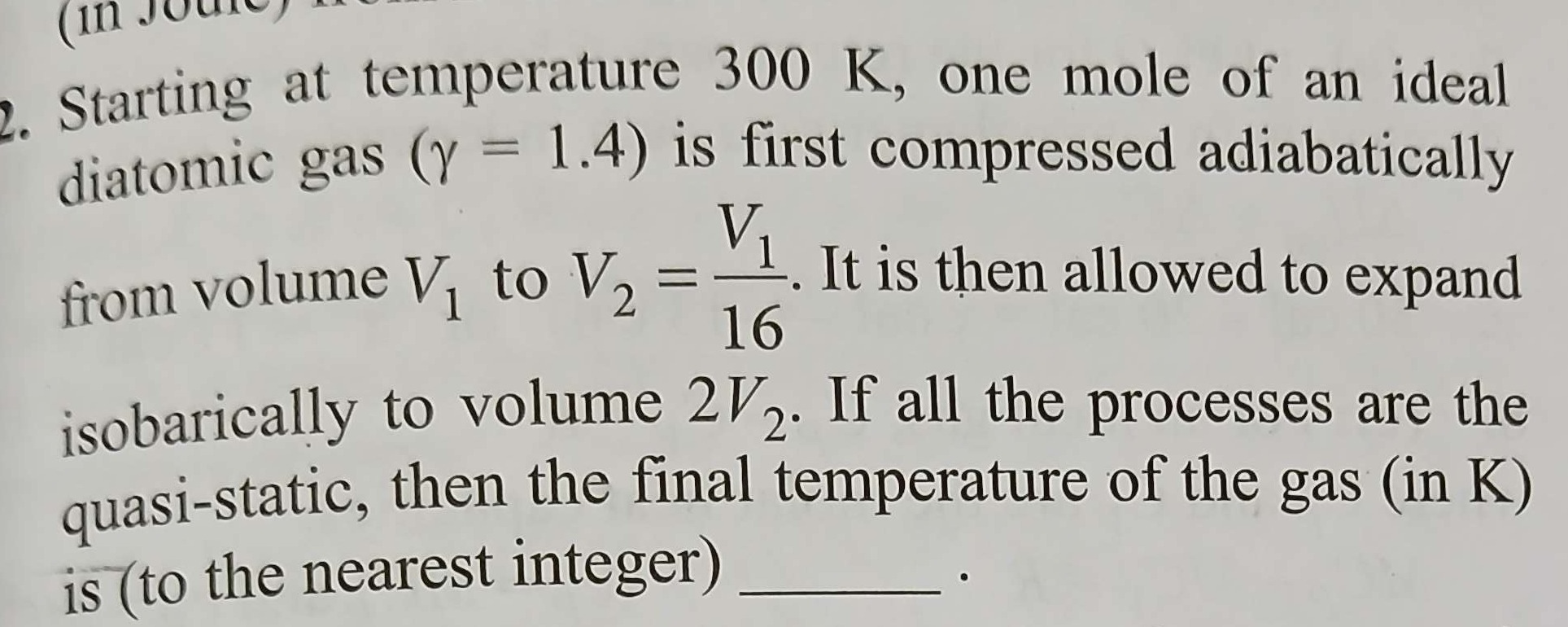
Answer
1819
Explanation
Solution
-
Adiabatic Compression:
TVγ−1=constant
For an adiabatic process,With initial T1=300K at volume V1 and final volume V2=16V1, the temperature after compression is
T2=T1(V2V1)γ−1=300(16)0.4.Calculate (16)0.4=e0.4ln16≈e1.109≈3.031.
T2≈300×3.031≈909K.
Thus, -
Isobaric Expansion:
T3=T2×2≈909×2≈1818K.
Under constant pressure, temperature is directly proportional to volume. When volume is doubled from V2 to 2V2:Rounding to the nearest integer gives 1819 K.
