Question
Question: 2-phenylethanol may be prepared by the reaction of phenylmagnesium bromide with: A. HCHO B. \(C{...
2-phenylethanol may be prepared by the reaction of phenylmagnesium bromide with:
A. HCHO
B. CH3CHO
C. CH3COCH3
D. 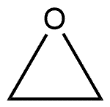
Solution
2-phenylethanol can also be known by the name phenethyl alcohol. It is generally an organic compound consists a phenethyl group attached with alcohol and show as C6H5CH2CH2OH. It is a colorless liquid and has less solubility in water as compared with organic solvents.
Complete Step by step solution: Phenyl magnesium bromide is represented by C6H5MgBr it is kept in the category of organometallic compounds. It is known as Grignard reagent. It is a strong nucleophile as well as a strong base. It can easily abstract acidic protons. With carbon dioxide phenyl magnesium bromide reacts to give benzoic acid after an acidic workup. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF).
-Given reaction is an example of nucleophilic reaction; these are those chemical addition reactions in which a nucleophile forms a sigma bond with an electron deficient species. In this reaction nucleophilic attack is done on epoxide ring which opens the epoxide ring and the reaction can be shown as follows:
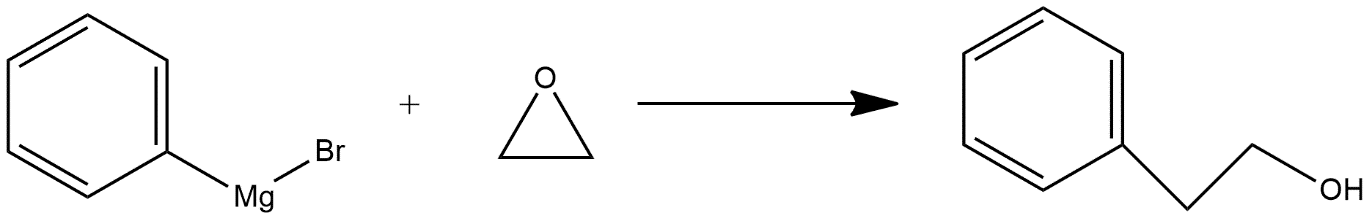
Hence we can say that option D is the correct answer.
Note: Grignard reagents are one of the most useful organometallic compounds. They exhibit strong nucleophilic qualities and also have the ability to form new carbon-carbon bonds; it is generally represented as an organomagnesium compound with the chemical formula R-MgX where R may be any alkyl or aryl group while X is halogen.
