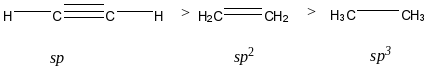Question
Question: 2-methyl-1,3-cyclohexandione is more acidic than cyclohexanone. A.True B.False...
2-methyl-1,3-cyclohexandione is more acidic than cyclohexanone.
A.True
B.False
Solution
We know that an acid is the substance that can release protons. The tendency of a compound to act as proton donor is termed as acidity. There are many factors on which acidity depends, such as electronegativity, hybridization, inductive effect.
Complete step by step answer:
Here, we have to compare the acidity of 2-methyl-1,3-cyclohexandione and cyclohexanone. Let’s first draw the structures of both the compounds.
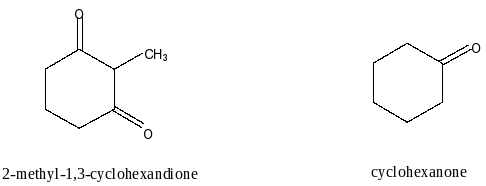
We know that acidity of a compound depends on the number of carbonyl groups in the compound. More the number of carbonyl groups results in more acidic strength of the compound. The reason for this is the carbonyl group is an electron withdrawing group (EWG). Due to the withdrawal of electrons, the donation of protons is easier.
Here, In 2-methyl-1,3-cyclohexanedione two carbonyl groups are present and in cylohexaneone one carboxyl group present. So, the acidity of 2-methyl-1,3-cyclohexanedione is more than cyclohexanedione. Therefore, 2-methyl-1,3-cyclohexandione is more acidic than cyclohexanone.
Hence, the correct answer is option A.
Additional Information:
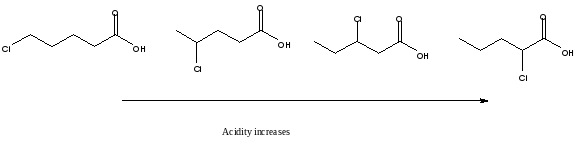
Always remember that the distance of the halogen atom to the carbonyl group affects the acidity of the compound. The closer the electronegative atom to the hydrogen, the stronger the acid is.
Note:
If the acidity of a set of organic unsaturated compounds is compared, always remember that we have to check the hybridization of the carbon atoms involved. More the s-character on the carbon atom indicates more electronegativity which again results in more acidity.