Question
Question: 2 kg water at \(100^\circ C\) and \(2.5kg\) water which is at \(50^\circ C\) is kept in two identica...
2 kg water at 100∘C and 2.5kg water which is at 50∘C is kept in two identical containers A and B respectively of water equivalent 0.5kg . If the water of the container A is placed poured into the container B the final temperature of mixture is T1 and if the water of the container B is poured into the container A the final temperature is T2 (heat loss is negligible)
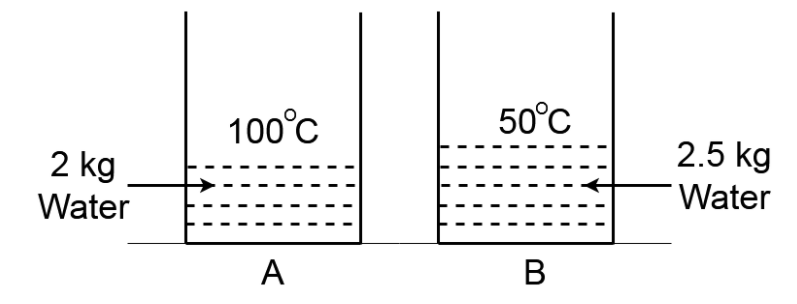
This question has multiple correct options
(A). T1=T2
(B). T2−T1=5
(C). T2=75∘C
(D). T1=75∘C
Solution
You can start by defining what water equivalent is and its importance in reaching the solution. Then write the equation for heat, i.e. Q=mcΔT . Then mention how heat lost and gained will be the same when the water in the containers is mixed. Used this concept to calculate the value of T1 and T2 . Compare the values of T1 and T2 to reach the solution.
Complete step-by-step answer :
Before attempting the solution, we should first discuss what water equivalent means.
The water equivalent of a specific body is the amount of water for which the heat evolved or absorbed will be the same as the amount of heat required to raise the body to the same temperature. To better understand it, consider a body, let’s take a ball as reference. So imagine we want to raise the temperature of the ball by X∘ by supplying it H amount of energy. So the water equivalent of this ball will be the amount of water whose temperature will change by X∘ on supplying H amount of energy.
It means that when the water will be poured from container A to container B , the container B will have an extra amount of water which will be equal to the water equivalent of the container B as the temperature of B will rise through the same temperature as the water in it. The situation is also similar when water is poured from container B to container A .
We know that
Q=mcΔT
Here, Q= Heat lost or gained
m= Mass
c= Specific heat of the substance
ΔT= Difference in temperature
So in case of mixing two solutions, we have
Heat lost = Heat gained
So, when we pour water from A to B
⇒mAcΔtA=(mB+0.5)cΔTB
⇒2c(100−T1)=(mB+0.5)c(T1−50)
⇒200−2T1=3T1−150
⇒5T1=350
⇒T1=70∘C
When we pour water from B and A
⇒(mA+0.5)cΔtA=mBcΔTB
⇒(2+0.5)c(100−T2)=2.5c(T2−50)
⇒100−T2=T2−50
⇒2T2=150
⇒T2=75∘C
Hence, T2−T1=75−70=5∘C .
Hence, options B and C are the correct choices.
Note : The answer that we obtained in the solution may not be the same as what you initially thought. Most of us would think that the total amount of water and the heat energy will be the same, so the final temperature should be the same no matter which container is poured into the other. But this is a misconception as we found out above.
