Question
Question: Find the number of following functions which are macroscopic properties. Pressure, Volume, Enthalpy,...
Find the number of following functions which are macroscopic properties. Pressure, Volume, Enthalpy, Work, Heat, Gibbs energy, temperature, Internal energy, Entropy.
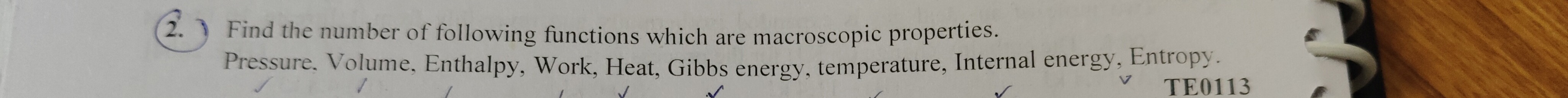
Answer
7
Explanation
Solution
Macroscopic properties are state variables of a system, measurable without considering microscopic details. Pressure, Volume, Enthalpy, Gibbs energy, Temperature, Internal energy, and Entropy are state functions, hence they are macroscopic properties. Work and Heat are energy transfers during a process (path functions), not properties of the system's state. Therefore, there are 7 macroscopic properties in the given list.
