Question
Question: Find sum of unpaired electrons in :- Atomic Nitrogen ; K$_3$[Fe(CN)$_6$] ; unstable S$_2$ molecule ...
Find sum of unpaired electrons in :-
Atomic Nitrogen ; K3[Fe(CN)6] ; unstable S2 molecule ; BaO2 ; Nitric oxide ; Ozone ; Dioxygen ; [Ti(H2O)6]Cl3 ; [MnCl6]3− ; [Mn(CN)6]3−
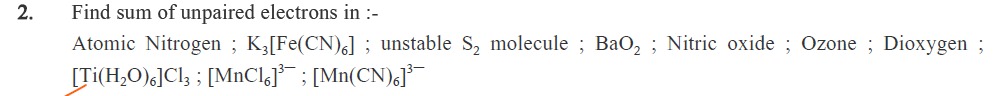
16 unpaired electrons
Solution
We'll determine the number of unpaired electrons species‐wise and then add them up.
Step‐by‐Step:
-
Atomic Nitrogen, N:
Configuration: 1s² 2s² 2p³ → Three electrons in 2p (each unpaired).
Unpaired electrons = 3 -
K₃[Fe(CN)₆]:
In [Fe(CN)6]3−, Fe is in +3 oxidation state ⇒ d5. Cyanide is a strong‐field ligand so the complex is low‐spin.
Configuration: t2g5 → One orbital must pair two electrons, leaving the other two orbitals singly occupied.
Unpaired electrons = 1 -
Unstable S₂ molecule:
MO theory (analogous to O₂) gives two electrons in degenerate π∗ orbitals.
Unpaired electrons = 2 -
BaO₂ (Barium peroxide):
The O₂²⁻ (peroxide ion) has two extra electrons compared to O₂ filling the π∗ orbitals. This leads to all electrons being paired.
Unpaired electrons = 0 -
Nitric Oxide, NO:
MO theory shows one unpaired electron.
Unpaired electrons = 1 -
Ozone, O₃:
Resonance forms yield a structure with all electrons paired.
Unpaired electrons = 0 -
Dioxygen, O₂:
Standard MO diagram for O₂ shows two unpaired electrons in the π∗ orbitals.
Unpaired electrons = 2 -
[Ti(H₂O)₆]Cl₃:
In [Ti(H2O)6]3+, Ti is in +3 oxidation state. Ti (atomic no. 22) loses three electrons so that d1 remains.
Unpaired electrons = 1 -
[MnCl₆]³⁻:
For [MnCl6]3−: 6 Cl⁻ (each –1) gives Mn oxidation state +3: d4. Chloride is a weak-field ligand → high-spin configuration.
High spin d4: three electrons in t2g and one electron in eg (all unpaired).
Unpaired electrons = 4 -
[Mn(CN)₆]³⁻:
Here, Mn is also +3 (d4), but CN⁻ is a strong-field ligand → low-spin configuration.
For low-spin d4: electrons fill t2g orbitals as: one electron in each orbital (3 unpaired) and the 4th electron pairs in one, leaving 2 unpaired electrons overall.
Unpaired electrons = 2
Total Sum:
3+1+2+0+1+0+2+1+4+2=16Each species’ unpaired electrons were determined via electronic configuration (using MO theory for diatomic molecules and ligand field theory for complexes). Their total is 16.
