Question
Question: What is number of moles of electrons gained by one mole of oxidizing agent in the following reaction...
What is number of moles of electrons gained by one mole of oxidizing agent in the following reaction?
Mn(aq)2++2ClO(aq)−⟶MnO2(s)+2ClO2(aq)
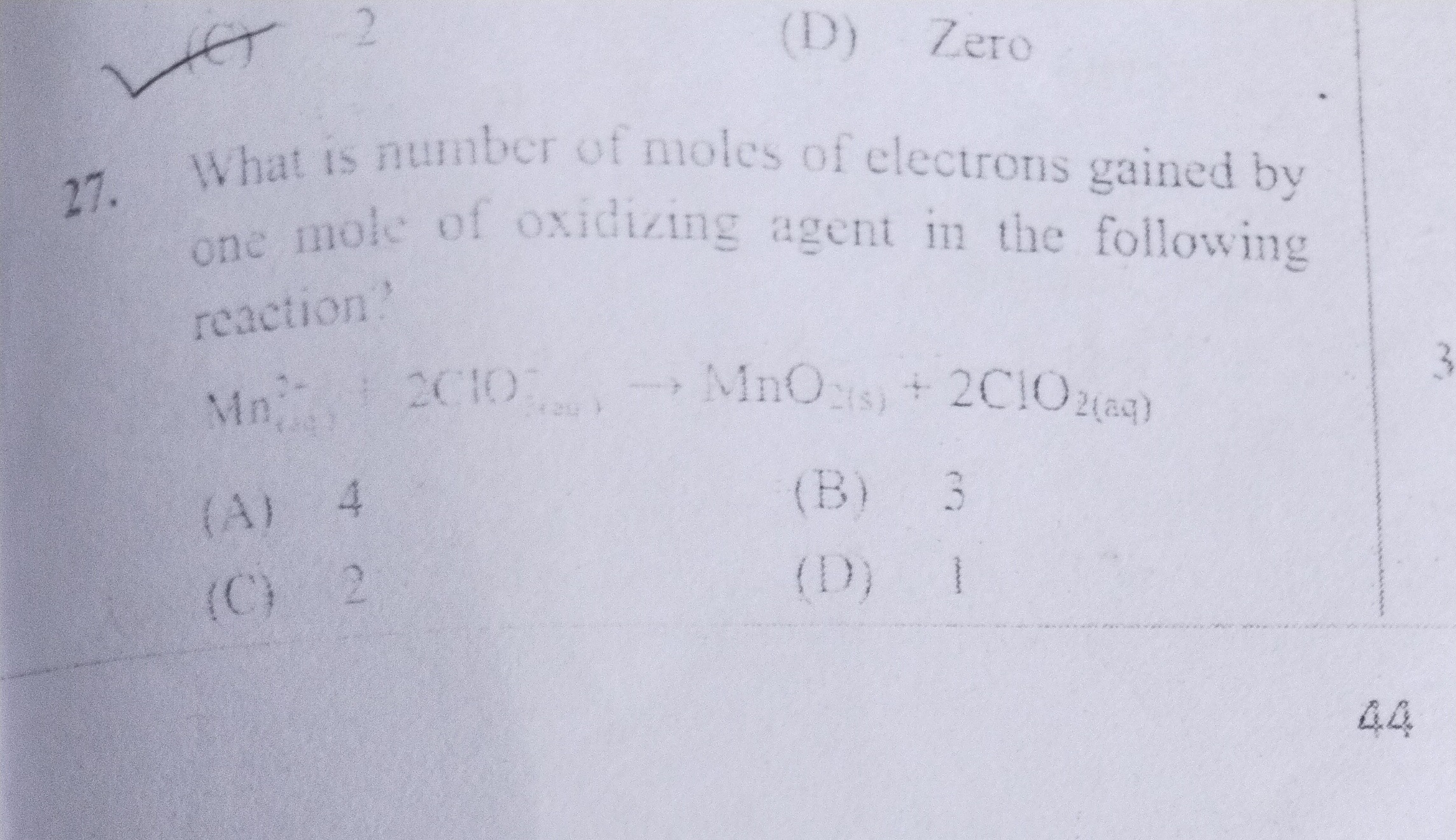
4
3
2
1
Zero
Solution
Solution:
-
In any redox reaction the oxidizing agent undergoes reduction (i.e. it gains electrons).
-
Check the oxidation‐states:
-
For Mn: In Mn²⁺ the oxidation state is +2; in MnO₂ (with O = –2) Mn is +4. This is an increase → oxidation.
-
For chlorine in ClO⁻: Let its oxidation number be x. Then:
x + (–2) = –1 ⟹ x = +1.
In ClO₂ the chlorine oxidation state (assuming oxygen remains –2) is:
x + 2(–2) = 0 ⟹ x = +4.
This is also an increase in oxidation state → oxidation.
-
-
Since both Mn²⁺ and ClO⁻ are oxidized, no species is being reduced; hence there is no oxidizing agent gaining electrons.
-
Answer: The number of moles of electrons gained by one mole of the oxidizing agent is 0.
Summary of Response:
-
Core Explanation:
Both Mn²⁺ and ClO⁻ undergo an increase in oxidation state (from +2 to +4 for Mn, and from +1 to +4 for Cl) so neither is reduced. Therefore, the oxidizing agent (the species that should gain electrons) is absent – no electrons are gained.
-
Answer: 0 (Zero electrons)
(Since none of the given options (A) 4, (B) 3, (C) 2, (D) 1 matches, the correct answer is 0.)
