Question
Question: The IUPAC name of the compound $CH_3-CH-C-CH_2CH_3$ $\quad | \quad |$ $CH_3 \quad CH_2$...
The IUPAC name of the compound
CH3−CH−C−CH2CH3 ∣∣ CH3CH2
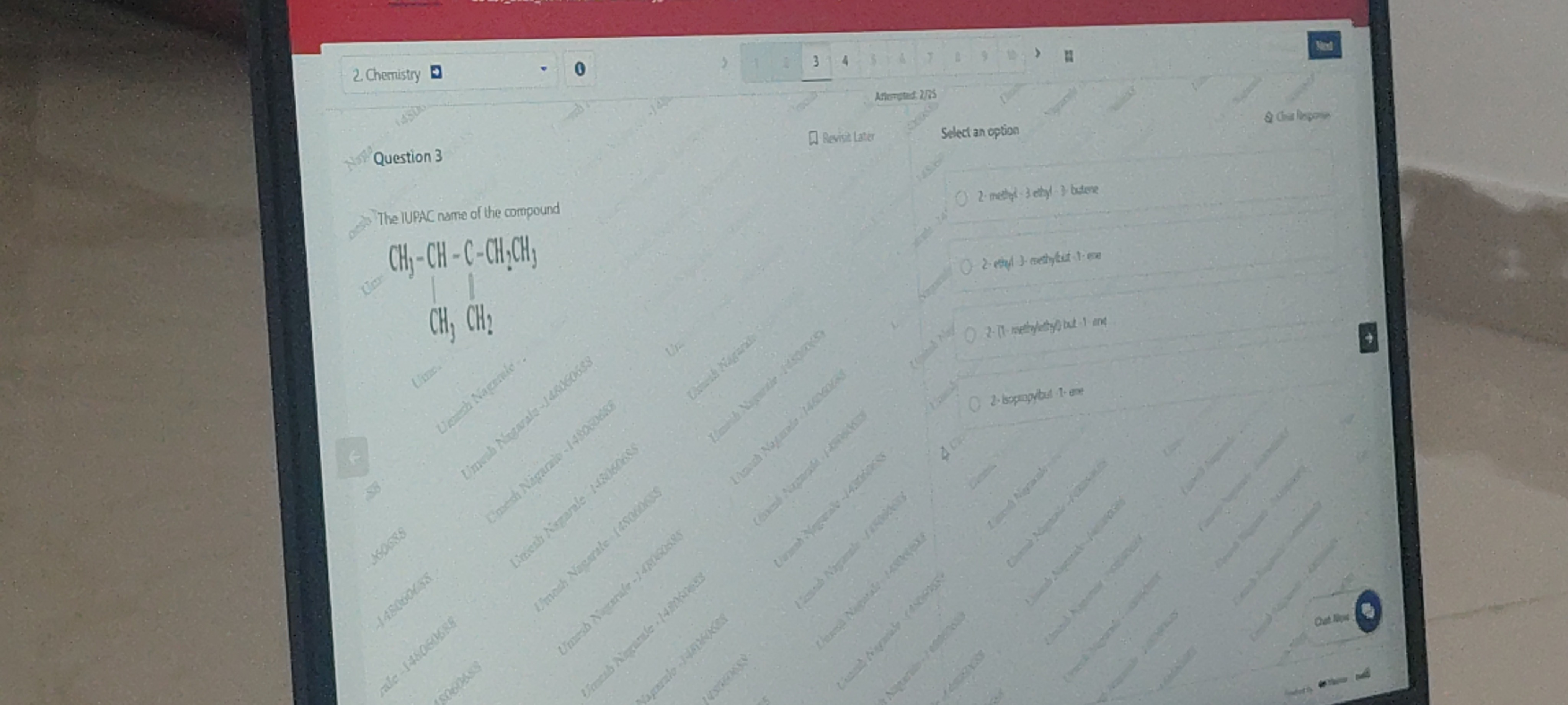
2-methyl-3 ethyl-3-butene
2-ethyl-3-methylbut-1-ene
2-(1-methylethyl)but-1-ene
2-Isopropylbut-1-ene
2-ethyl-3-methylbut-1-ene
Solution
To determine the IUPAC name of the compound, we first need to correctly interpret its structure.
The given structure is: CH3−CH−C−CH2CH3 ∣∣ CH3CH2
This representation indicates that:
- The
CH3group belowCHis a substituent on thatCH. So, it'sCH(CH3). - The
CH2group belowCindicates a double bond betweenCandCH2. So, it'sC=CH2. (If it were a single bond, the carbonCwould have only 3 bonds, which is impossible, or it would be written asCH2-CorC-CH2in a linear fashion).
Therefore, the expanded structure of the compound is: CH3−CH(CH3)−C(=CH2)−CH2CH3
Now, let's follow the IUPAC rules for naming alkenes:
-
Identify the longest continuous carbon chain that includes both carbon atoms of the double bond. Let's number the double bond carbons as C1 and C2, ensuring the double bond gets the lowest possible numbers. The double bond is
C=CH2. LetCH2be C1 andCbe C2.From C2, there are two branches extending:
- Branch A:
-CH2CH3(an ethyl group, 2 carbons) - Branch B:
-CH(CH3)CH3(an isopropyl group, 3 carbons)
Let's explore the possible parent chains containing the double bond:
-
Option 1 (Parent chain includes Branch A): If we include
-CH2CH3as part of the main chain, the chain would beCH2=C-CH2-CH3. Numbering:CH2=C-CH2-CH31 2 3 4This is a 4-carbon chain, so the parent alkane isbut-1-ene. The substituent on this chain is the-CH(CH3)CH3(isopropyl) group, which is attached to C2. Name: 2-isopropylbut-1-ene (or 2-(1-methylethyl)but-1-ene). This chain has 1 substituent. -
Option 2 (Parent chain includes Branch B): If we include
-CH(CH3)CH3as part of the main chain, the chain would beCH2=C-CH(CH3)-CH3. Numbering:CH2=C-CH(CH3)-CH31 2 3 4This is also a 4-carbon chain, so the parent alkane isbut-1-ene. The substituents on this chain are:-CH2CH3(ethyl group) attached to C2.-CH3(methyl group) attached to C3. Name: 2-ethyl-3-methylbut-1-ene. This chain has 2 substituents.
- Branch A:
-
Choose the parent chain based on the number of substituents. Both Option 1 and Option 2 give a 4-carbon parent chain (
but-1-ene). When two chains of equal length are possible, the IUPAC rule states that the chain with the maximum number of substituents should be chosen as the parent chain. Option 1 has 1 substituent (isopropyl). Option 2 has 2 substituents (ethyl and methyl). Therefore, Option 2 is the correct choice for the parent chain. -
Name the substituents and arrange them alphabetically. The parent chain is
but-1-ene. The substituents areethylat position 2 andmethylat position 3. In alphabetical order, ethyl comes before methyl. -
Construct the full IUPAC name. Combining the substituent names with their positions and the parent chain name: 2-ethyl-3-methylbut-1-ene
Comparing this with the given options:
- ◯ 2-methyl-3 ethyl-3-butene (Incorrect double bond position and numbering)
- ◯ 2-ethyl-3-methylbut-1-ene (Matches our derived name)
- ◯ 2-(1-methylethyl)but-1-ene (This corresponds to 2-isopropylbut-1-ene, which was ruled out as it has fewer substituents on the parent chain)
- ◯ 2-Isopropylbut-1-ene (Same as above, ruled out)
The final answer is 2-ethyl-3-methylbut-1-ene.
Explanation of the solution (minimal): The compound is CH3−CH(CH3)−C(=CH2)−CH2CH3.
- Identify the longest carbon chain containing the double bond. Two 4-carbon chains are possible:
- Chain 1: CH2=C−CH2−CH3. This chain has one substituent (isopropyl) at C2.
- Chain 2: CH2=C−CH(CH3)−CH3. This chain has two substituents (ethyl at C2 and methyl at C3).
- According to IUPAC rules, if chains are of equal length, select the one with the maximum number of substituents. Chain 2 has 2 substituents, while Chain 1 has 1. Therefore, Chain 2 is the correct parent chain,
but-1-ene. - Number the parent chain to give the double bond the lowest possible locant (1).
- Identify and name the substituents: ethyl at C2 and methyl at C3.
- List substituents alphabetically: ethyl before methyl. The IUPAC name is 2-ethyl-3-methylbut-1-ene.
