Question
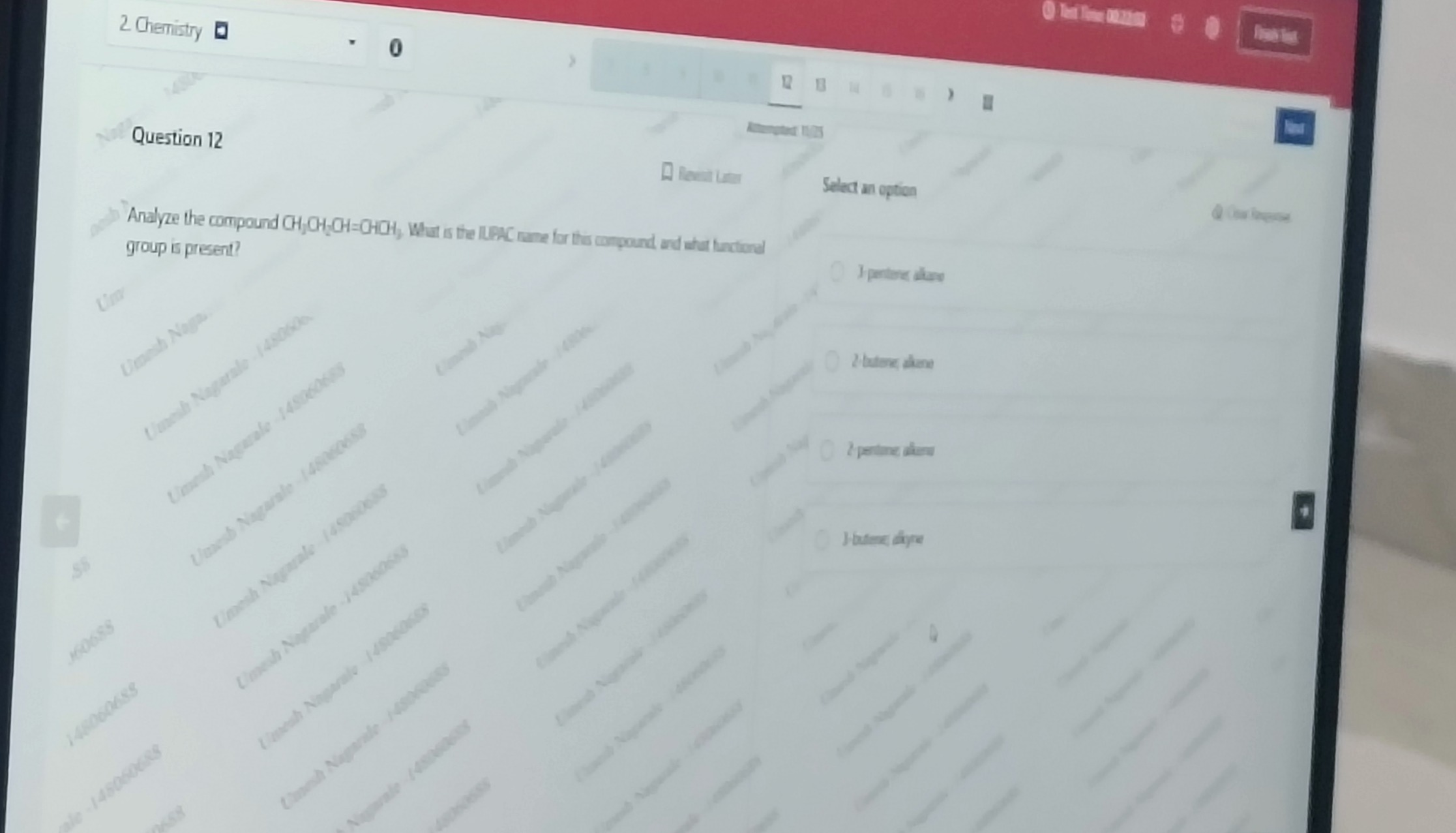
Question: Analyze the compound $CH_2CH_2CH=CHCH_3$. What is the IUPAC name for this compound, and what functio...
Analyze the compound CH2CH2CH=CHCH3. What is the IUPAC name for this compound, and what functional group is present?
A
3-pentene; alkane
B
2-butene; alkene
C
2-pentene; alkene
D
3-butene; alkyne
Answer
2-pentene; alkene
Explanation
Solution
The compound CH2CH2CH=CHCH3 is interpreted as CH3CH2CH=CHCH3. The longest carbon chain containing the double bond has 5 carbons, so the root is "pent". The presence of a carbon-carbon double bond indicates it's an alkene, so the suffix is "-ene". Numbering the chain from the end that gives the double bond the lowest possible number (from right to left) places the double bond at position 2. Thus, the IUPAC name is 2-pentene, and the functional group is an alkene.
