Question
Question: 1L of a gas is at a pressure of \[{{10}^{-6}}\]of Hg at \[{{25}^{o}}C\]. How many molecules are pres...
1L of a gas is at a pressure of 10−6of Hg at 25oC. How many molecules are present?
(A)- 3.2×106
(B)- 3.2×1013
(C)- 3.2×1010
(D)- 3×104
Solution
We can calculate the number of molecules using the ideal gas equation. In the above question we are given the pressure, volume and temperature of the gas.
PV=nRT(Ideal gas equation)
Number of molecules …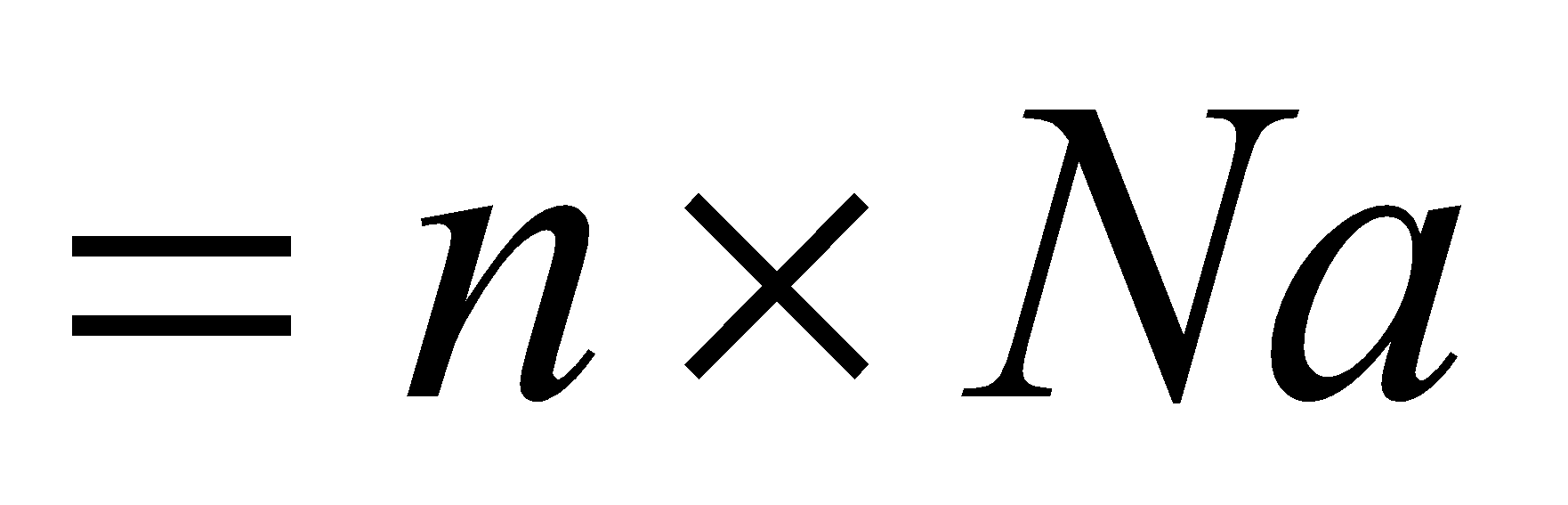 …where Na is Avogadro number
…where Na is Avogadro number
Complete step by step solution:
Let’s look at the answer
The formula for the ideal gas equation is:
PV=nRT…..Where P is pressure, V is volume, R is gas constant, n is number of moles, and T is absolute temperature.
On transforming the equation for the number of moles, n, we get
n=RTPV……..eq1
Now, it is given in the question that
P=10−6of Hg =76010−6atm
V=1L
R=0.0821Latm/molK
T=25oC=25+273=298K
Now, put the values of P, V, T in eq1
We get,
n=760×0.0821×29810−6×1……..eq2
Now, using the formula for number of molecules
We get,
Number of molecules ……where Na is Avogadro number
On putting the values of n from eq2 and Na=6.02×1023
We get the number of molecules as:
Number of molecules =\dfrac{{{10}^{-6}}\times 6.02\times {{10}^{23}}}{760\times 0.0821\times 298}$$$$=3.2\times {{10}^{13}}
So, the number of molecules of the given gas are=3.2×1013
Hence, our final answer is option (B).
Note: The temperature should be converted into kelvin. The pressure should be taken into atmospheres. The value of R should be taken according to the units of P and V. If temperature is not given then take 298K as the standard temperature.
