Question
Question: Circle represents most basic atoms in these molecule. Which of the following is correct representati...
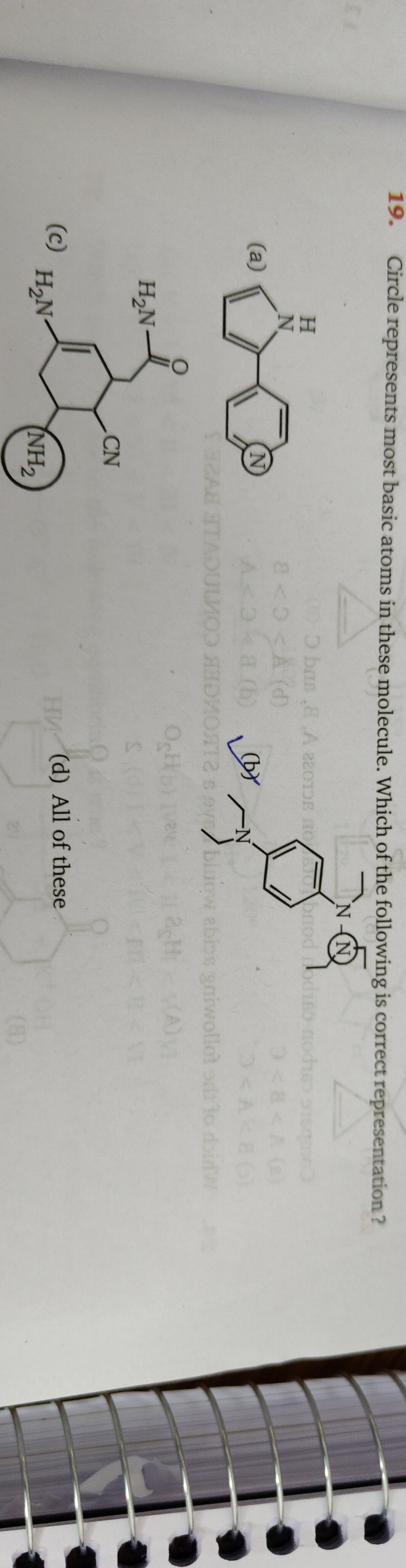
Circle represents most basic atoms in these molecule. Which of the following is correct representation?
H₂N
All of these.
(d) All of these.
Solution
The question asks to identify the correct representation where a circle highlights the most basic atom in the given molecule. Basicity is determined by the availability of lone pair electrons for donation.
-
Option (a): The molecule is 2,2'-bipyridine. The circled atom is a nitrogen atom in a pyridine ring. Pyridine nitrogens have lone pairs in sp² hybrid orbitals, which are not involved in resonance and are thus basic. Both nitrogen atoms in 2,2'-bipyridine are equivalent and are the most basic sites. The circled atom correctly represents a most basic atom.
-
Option (b): The molecule is N,N-diethyl-N'-phenyl-1,4-phenylenediamine. The circled nitrogen is part of a -N(Et)₂ group attached to a phenyl ring. This is a tertiary amine. The other nitrogen is an aniline-type nitrogen, which is directly attached to a phenyl ring and a phenyl group. The lone pair on the aniline nitrogen is delocalized into the phenyl ring, making it less basic. The lone pair on the tertiary amine nitrogen is not significantly delocalized and is more available for donation, especially with the electron-donating ethyl groups. Therefore, the circled nitrogen is the most basic atom.
-
Option (c): The molecule contains an amine group (-NH₂), a nitrile group (-CN), and an amide group (-CONH₂). The circled atom is the nitrogen in the -NH₂ group.
- The nitrogen in the amine group (-NH₂) is sp³ hybridized and has a localized lone pair, making it basic.
- The nitrogen in the nitrile group (-CN) is sp hybridized. Its lone pair is in an sp orbital, which has higher s-character than sp³ orbitals, making it less basic.
- The nitrogen in the amide group (-CONH₂) has its lone pair delocalized into the carbonyl group through resonance, making it very weakly basic. Thus, the circled nitrogen in the amine group is the most basic atom.
Since the circled atom in each of the options (a), (b), and (c) correctly represents the most basic atom in its respective molecule, option (d) "All of these" is the correct answer.
