Question
Question: The enthalpy change when 1 g of water is frozen at 0°C ($\Delta_{fus}H^0$ = 1.435 kcl/mol) is...
The enthalpy change when 1 g of water is frozen at 0°C (ΔfusH0 = 1.435 kcl/mol) is
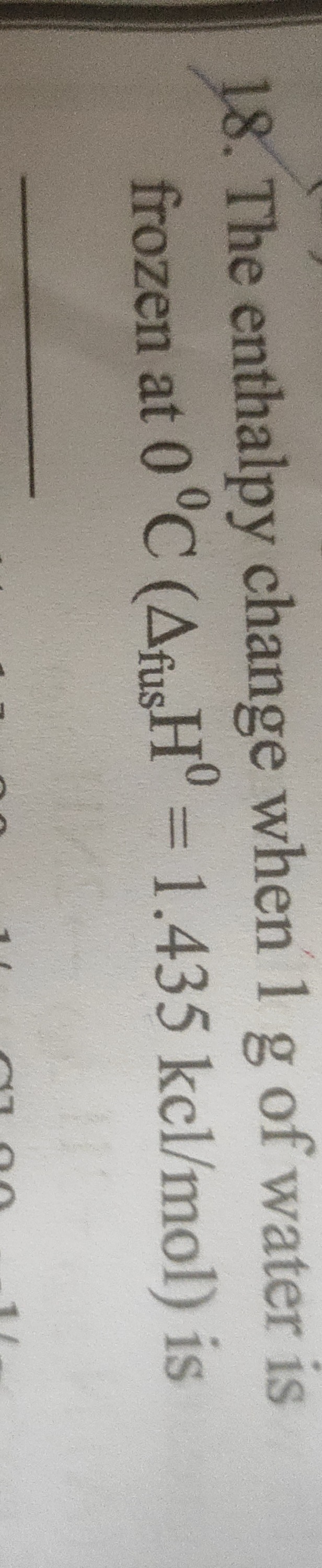
Answer
-0.08 kcal
Explanation
Solution
-
Convert grams to moles:
Moles of water = 18g/mol1g≈0.0556mol. -
Calculate enthalpy change (freezing):
ΔH=−0.0556mol×1.435kcal/mol≈−0.0797kcal
For freezing, the enthalpy change is the negative of the fusion value.This approximately equals −0.08kcal.
Core Explanation:
Convert 1 g water to moles (1/18 mol) and multiply by ΔfusH0, then take the negative sign because freezing is exothermic.
