Question
Question: Hydrogen atom from $n^{th}$ energy state comes to the ground state by emitting a photon of wavelengt...
Hydrogen atom from nth energy state comes to the ground state by emitting a photon of wavelength λ. The value of ‘n’ will be: (R: Rydberg constant)
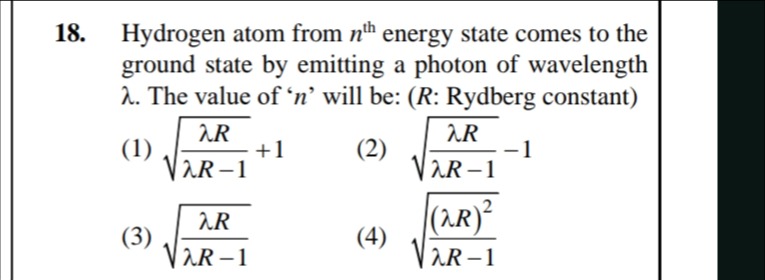
λR−1λR+1
λR−1λR−1
λR−1λR
λR−1(λR)2
λR−1λR
Solution
The problem asks us to find the principal quantum number 'n' of the initial energy state from which a hydrogen atom transitions to the ground state, emitting a photon of wavelength λ.
We use the Rydberg formula for the wavelength of light emitted during an electronic transition in a hydrogen atom: λ1=R(nf21−ni21) where:
- λ is the wavelength of the emitted photon.
- R is the Rydberg constant.
- ni is the principal quantum number of the initial energy state.
- nf is the principal quantum number of the final energy state.
Given:
- The atom comes from the nth energy state, so ni=n.
- The atom comes to the ground state, so nf=1.
Substitute these values into the Rydberg formula: λ1=R(121−n21) λ1=R(1−n21)
Now, we need to solve for 'n'. Multiply both sides by λ: 1=λR(1−n21)
Divide both sides by λR: λR1=1−n21
Rearrange the equation to isolate n21: n21=1−λR1
Combine the terms on the right-hand side: n21=λRλR−1
Take the reciprocal of both sides to find n2: n2=λR−1λR
Finally, take the square root of both sides to find 'n': n=λR−1λR
Comparing this result with the given options, we find that it matches option (3).
