Question
Question: Find wavelength of photon emitted during its transition from 4E/3 level to E level, if $\lambda$ is ...
Find wavelength of photon emitted during its transition from 4E/3 level to E level, if λ is the wavelength emitted during transition from 2E level to E level in the following diagram :- (Here E represents the energy of shell)
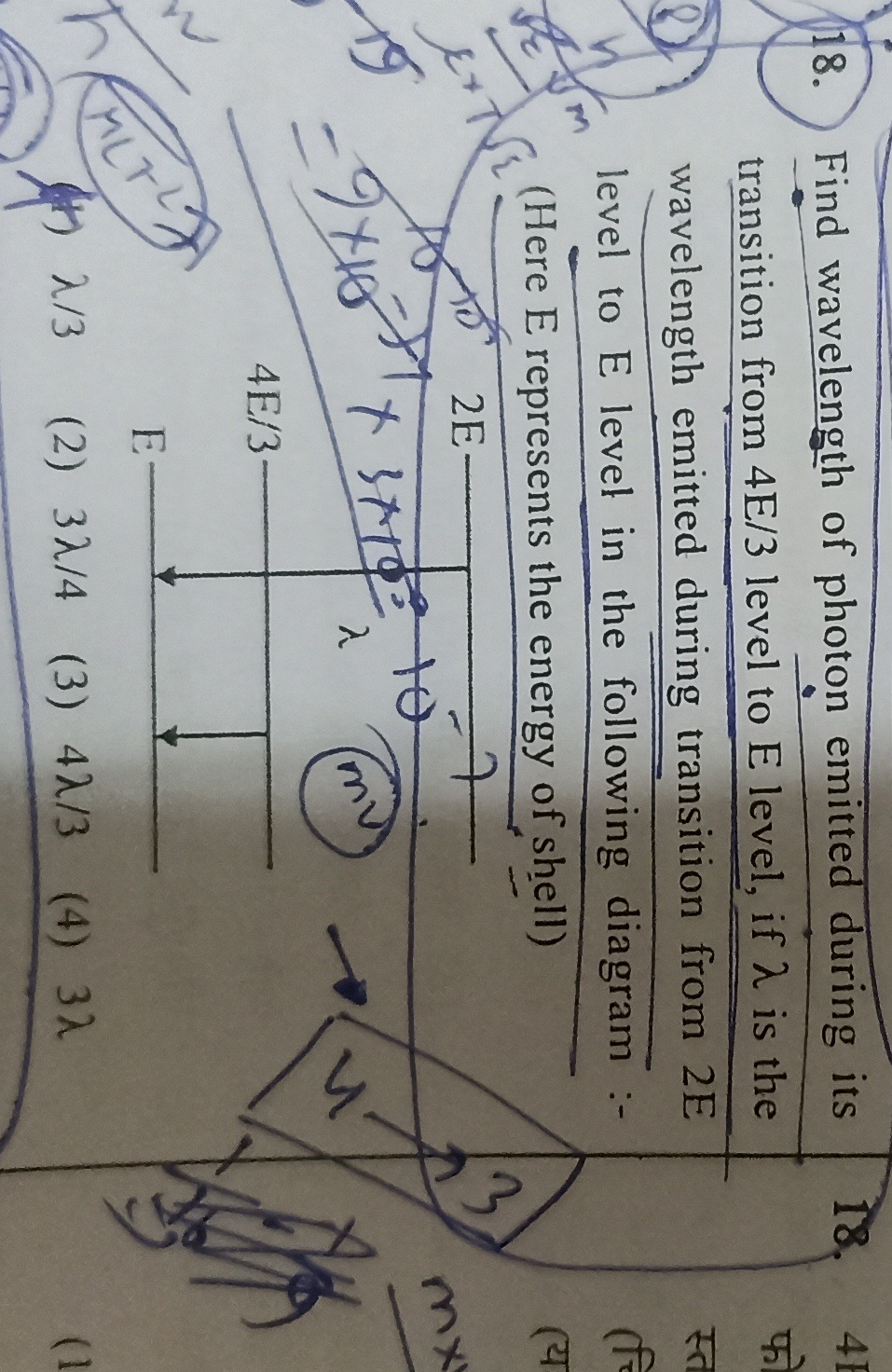
λ/3
3λ/4
4λ/3
3λ
3λ
Solution
To find the wavelength of the emitted photon, we use the relationship between energy difference and wavelength:
E=λhc
where E is the energy of the photon, h is Planck's constant, c is the speed of light, and λ is the wavelength.
Given:
-
A photon of wavelength λ is emitted during a transition from the 2E level to the E level. The energy difference for this transition (ΔE1) is: ΔE1=2E−E=E So, we can write: E=λhc (Equation 1)
-
We need to find the wavelength (let's call it λ′) of the photon emitted during the transition from the 4E/3 level to the E level. The energy difference for this transition (ΔE2) is: ΔE2=34E−E=34E−3E=3E So, we can write: 3E=λ′hc (Equation 2)
Now, we need to express λ′ in terms of λ. From Equation 1, we can express hc: hc=Eλ
Substitute this expression for hc into Equation 2: 3E=λ′Eλ
Since E is a non-zero energy value, we can cancel E from both sides of the equation: 31=λ′λ
Now, solve for λ′: λ′=3λ
Thus, the wavelength of the photon emitted during the transition from the 4E/3 level to the E level is 3λ.
