Question
Question: 18....
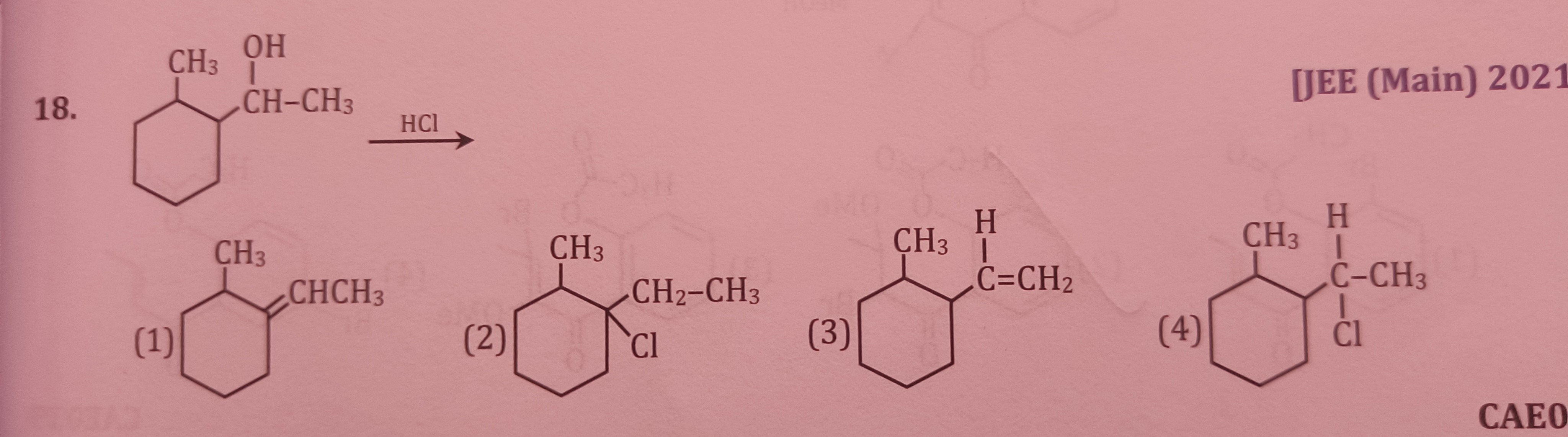
Cyclohexane ring with a CH3 group and a -CH(Cl)CH3 group on adjacent carbons.
Cyclohexane ring with a CH3 group and a -CH2CH2Cl group on adjacent carbons.
Cyclohexane ring with a CH3 group and a -C(CH3)2Cl group on adjacent carbons.
Cyclohexane ring with a CH3 group and a -C(Cl)CH3 group on adjacent carbons.
4
Solution
The reaction of a secondary alcohol with HCl proceeds via an SN1 mechanism, involving protonation of the hydroxyl group, followed by the loss of water to form a carbocation. The initial carbocation formed is secondary. This secondary carbocation can undergo rearrangement to form a more stable tertiary carbocation. The most plausible rearrangement involves a methyl shift from the adjacent ring carbon to the carbocation center, forming a tertiary carbocation. This tertiary carbocation is then attacked by chloride ion. However, given the options, option (4) represents the product without rearrangement (1-(1-chloroethyl)-2-methylcyclohexane), which is also a possible product, especially if steric hindrance or specific reaction conditions disfavor rearrangement. Assuming option (4) is the intended correct answer, it implies direct substitution or a rearrangement pathway that is not immediately obvious from standard mechanisms that would lead to the other options.
