Question
Question: What volume (in ml) of 0.8 M $AlCl_3$ solution to get solution of chloride ion concentration equal t...
What volume (in ml) of 0.8 M AlCl3 solution to get solution of chloride ion concentration equal to 0.6 M ?
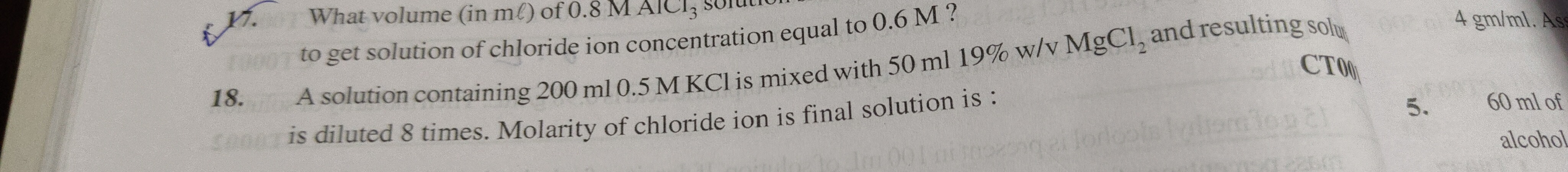
250 ml
Solution
The question asks for a specific volume of a concentrated AlCl3 solution required to prepare a solution with a desired chloride ion concentration. To obtain a numerical answer for volume, we must assume a final target volume for the solution to be prepared. A standard assumption in such cases is to prepare 1 liter (1000 ml) of the final solution.
-
Determine moles of chloride ions needed in the final solution: We want a final solution with a chloride ion concentration of 0.6 M. If we assume the final volume is 1 L (1000 ml): Moles of Cl− needed=Molarity×Volume (in L)=0.6 mol/L×1 L=0.6 mol
-
Relate moles of chloride ions to moles of AlCl3: Aluminum chloride (AlCl3) dissociates in water as follows: AlCl3(aq)→Al3+(aq)+3Cl−(aq) This means 1 mole of AlCl3 produces 3 moles of Cl− ions. Therefore, to get 0.6 moles of Cl−, we need: Moles of AlCl3 required=3Moles of Cl− needed=30.6 mol=0.2 mol
-
Calculate the volume of the 0.8 M AlCl3 stock solution: We have a stock solution of 0.8 M AlCl3. To find the volume containing 0.2 moles of AlCl3: Volume of AlCl3 solution=Molarity of AlCl3Moles of AlCl3 required=0.8 mol/L0.2 mol=0.25 L Converting to milliliters: 0.25 L×1000 ml/L=250 ml
