Question
Question: The major product of the following reaction is:...
The major product of the following reaction is:
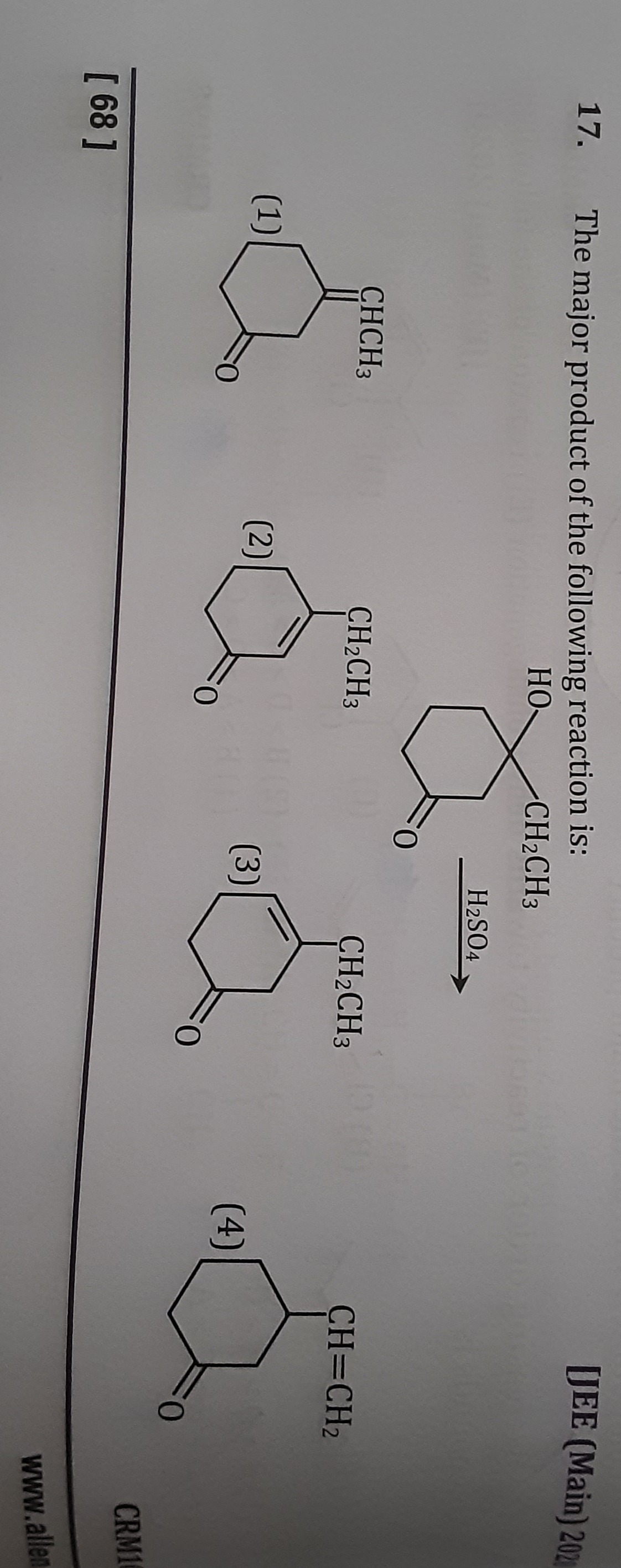
Cyclohexanone with =CHCH3 at position 2
Cyclohexenone with =C(CH2CH3) at position 2 and a double bond in the ring
Cyclohexenone with a double bond in the ring and CH2CH3 attached to the double bond
Cyclohexanone with =CH-CH2 at position 2
Cyclohexanone with =CHCH3 at position 2
Solution
The reaction involves the acid-catalyzed dehydration of a tertiary alcohol, specifically 2-ethyl-2-hydroxycyclohexanone. The mechanism proceeds through the following steps:
-
Protonation of the hydroxyl group: The sulfuric acid (H2SO4) protonates the hydroxyl group (-OH) of the alcohol, converting it into a good leaving group (-OH2+).
-
Formation of a carbocation: The protonated hydroxyl group departs as a water molecule (H2O), generating a tertiary carbocation at the carbon atom adjacent to the carbonyl group.
-
Elimination of a proton: The carbocation can undergo deprotonation from an adjacent carbon atom to form an alkene. In this case, protons can be removed from either the adjacent ring carbon (C3) or from the ethyl group.
-
Elimination towards C3 (ring carbon): Removal of a proton from C3 leads to the formation of an exocyclic double bond between C2 and C3. This results in 2-ethylidenecyclohexanone.
-
Elimination from the ethyl group: Removal of a proton from the -CH2- part of the ethyl group (-CH2CH3) would form a double bond between C2 and the first carbon of the ethyl group, yielding 2-(1-propenyl)cyclohexanone.
-
Considering the provided options and the typical outcomes of such reactions, the formation of the exocyclic alkene, 2-ethylidenecyclohexanone, is the major product. This corresponds to option (1).
