Question
Question: $C_6H_5$ CO $NHCH_3$ can be converted into $C_6H_5CH_2NHCH_3$ by...
C6H5 CO NHCH3 can be converted into C6H5CH2NHCH3 by
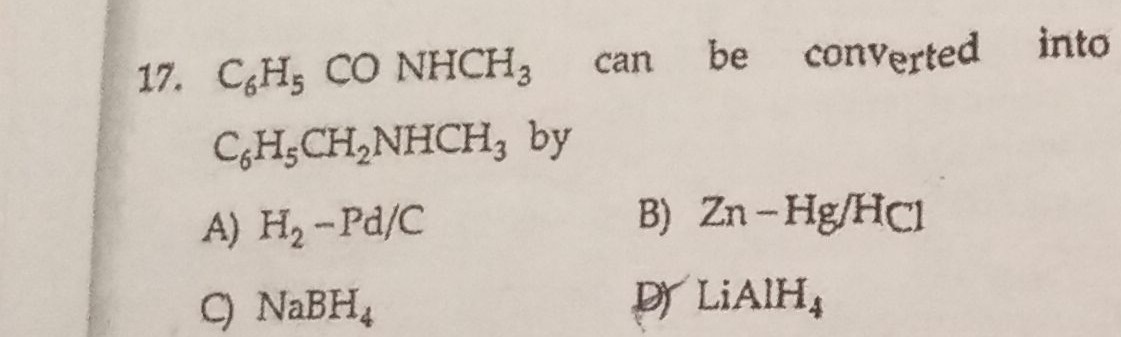
A
H2−Pd/C
B
Zn-Hg/HCl
C
NaBH4
D
LiAlH4
Answer
D) LiAlH4
Explanation
Solution
The reagent required must reduce the amide carbonyl (–CO–) in C6H5CO–NHCH3 to a CH2 group. Among the listed reagents, LiAlH4 is a strong hydride donor that effectively reduces amides to amines.
LiAlH4 reduces N-methylbenzamide (C6H5CO–NHCH3) to N-methylbenzylamine (C6H5CH2–NHCH3), whereas the other reagents are either too mild (NaBH4), require catalytic hydrogenation conditions (H2–Pd/C), or are used for Clemmensen reductions (Zn–Hg/HCl) which are not effective for amide groups.
