Question
Question: 16. Predict the major products (including stereochemistry) when R-2-Butanol reacts with the followin...
- Predict the major products (including stereochemistry) when R-2-Butanol reacts with the following reagents. (a) PBr₃
(b) SOCl₂/
(c) Lucas reagent (d) TsCl / pyridine, then NaI
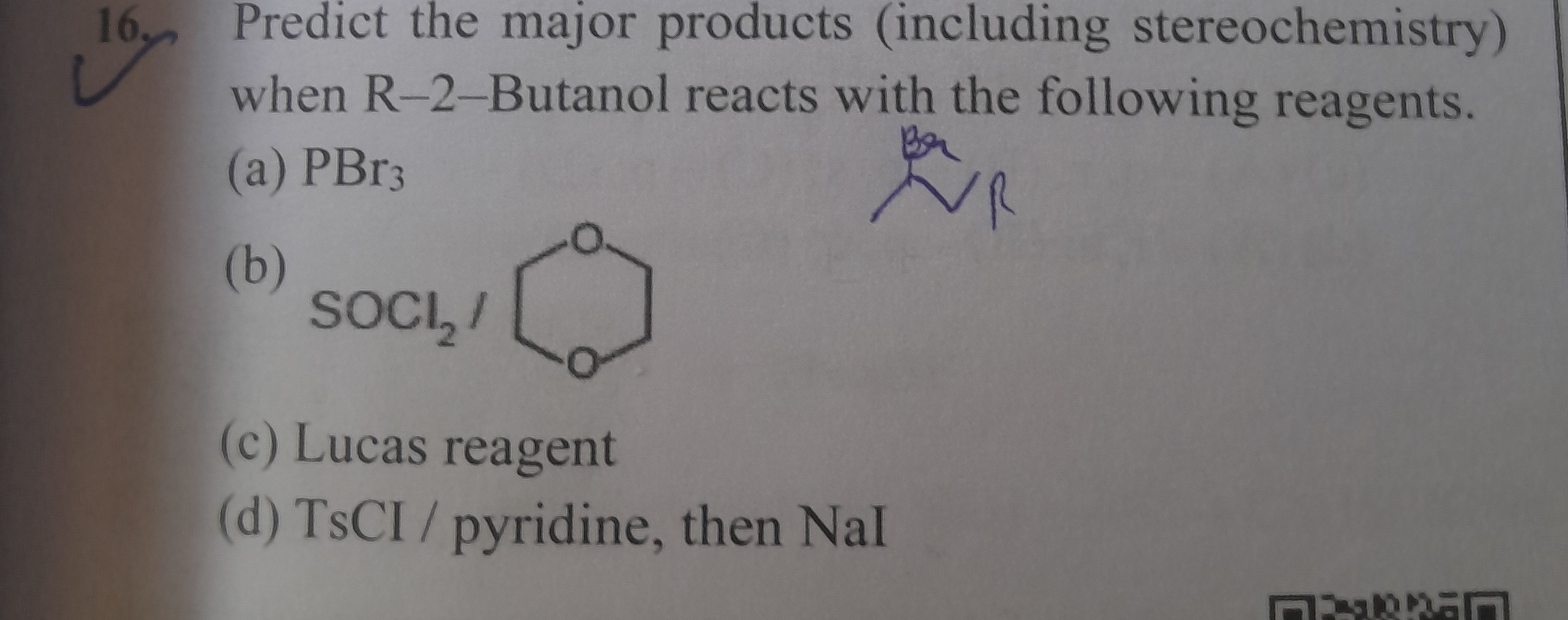
Answer
(a) (S)-2‑Bromobutane
(b) (S)-2‑Chlorobutane
(c) Racemic 2‑Chlorobutane
(d) (S)-2‑Iodobutane
Explanation
Solution
Solution:
Let the starting alcohol be (R)-2-butanol. Recall that:
- SN2 reactions (e.g. with PBr₃ and when using tosylate → iodide) involve inversion of configuration.
- SN1 reactions (e.g. with Lucas reagent) proceed via a carbocation so that the chiral center becomes racemic.
Now, step‐by-step:
(a) With PBr₃:
- PBr₃ converts the –OH into a bromide via an SN2 mechanism.
- Thus, (R)-2-butanol gives inversion yielding (S)-2‑bromobutane.
(b) With SOCl₂ in dioxane:
- Thionyl chloride generally reacts by forming a chlorosulfite intermediate and then substitution occurs via an SN2-like pathway.
- Hence, (R)-2-butanol is converted to (S)-2‑chlorobutane (inversion).
(c) With Lucas reagent (ZnCl₂/HCl):
- Lucas reagent reacts via an SN1 mechanism on secondary alcohols, forming a planar carbocation.
- This leads to a racemization of the center so the product is 2‑chlorobutane (racemic mixture).
(d) With TsCl/pyridine then NaI:
- First, TsCl converts the –OH into a tosylate (which retains the configuration; the stereochemistry is that of the starting alcohol).
- Then, NaI displaces the tosylate via an SN2 process causing inversion.
- Overall, (R)-2-butanol is converted to (S)-2‑iodobutane.
Minimal Explanation:
For (a) and (d): SN2 gives inversion (R becomes S). For (b): SOCl₂ in dioxane also proceeds via an SN2-like mechanism (inversion, S product). For (c): Lucas reagent involves an SN1 mechanism yielding a racemic mixture.
