Question
Question: In the given pair identify most acidic compound in (A) and (B). Most basic in (C) and (D)....
In the given pair identify most acidic compound in (A) and (B). Most basic in (C) and (D).
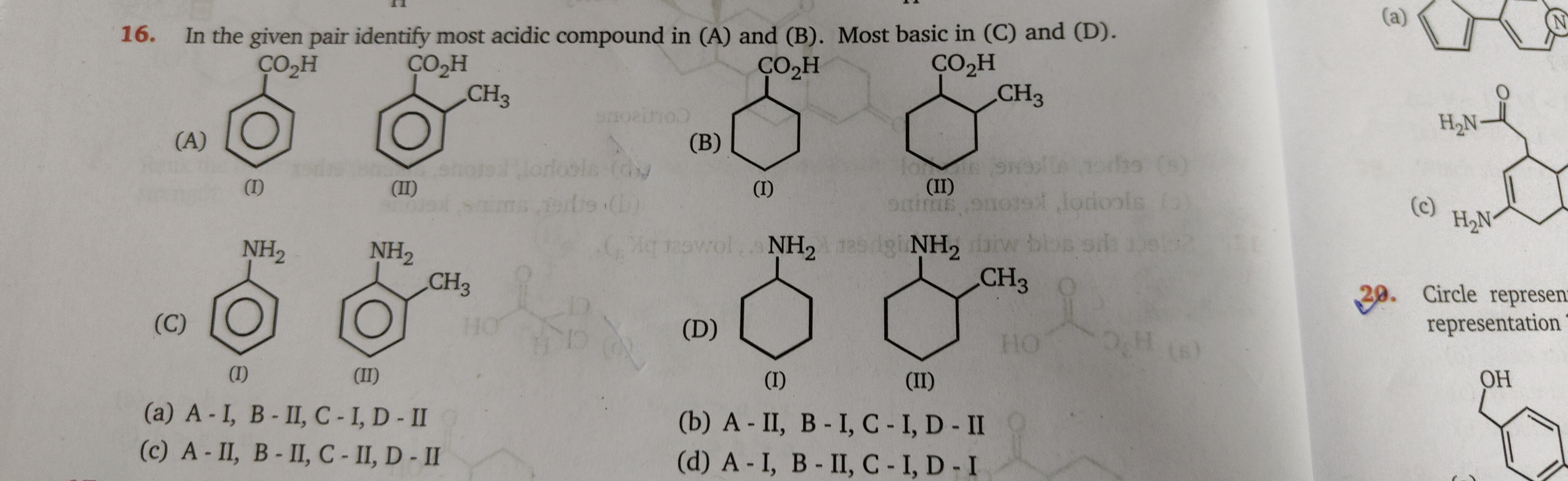
A-I, B-II, C - I, D - II
A - II, B - I, C - I, D - II
A-II, B-II, C - II, D - II
A-1, B-II, C-I, D-I
A - II, B - I, C - I, D - II
Solution
To determine the most acidic compound in pairs (A) and (B), we analyze the effects of substituents on the stability of the carboxylate anion. For basicity in pairs (C) and (D), we examine the availability of the lone pair on the nitrogen atom.
Acidity:
-
Pair (A): Benzoic acid (I) vs. o-toluic acid (II)
- Benzoic acid (I) has a -COOH group attached to a benzene ring.
- o-Toluic acid (II) has a -COOH group and a -CH₃ group at the ortho position on the benzene ring.
- The methyl group (-CH₃) is electron-donating (+I effect), which destabilizes the carboxylate anion, thus decreasing acidity.
- However, at the ortho position, steric hindrance from the methyl group causes the -COOH group to twist out of the plane of the benzene ring. This reduces the resonance stabilization of the carboxylate anion. While reduced resonance normally decreases acidity, the net effect of the ortho-methyl group in o-toluic acid leads to increased acidity compared to benzoic acid. (pKa of benzoic acid = 4.20, pKa of o-toluic acid = 3.91).
- Therefore, o-toluic acid (A-II) is more acidic.
-
Pair (B): Cyclohexanecarboxylic acid (I) vs. 2-methylcyclohexanecarboxylic acid (II)
- Cyclohexanecarboxylic acid (I) is an aliphatic carboxylic acid.
- 2-methylcyclohexanecarboxylic acid (II) has a methyl group at the alpha-carbon.
- The methyl group is electron-donating (+I effect). This increases electron density, destabilizing the carboxylate anion and decreasing acidity.
- Therefore, cyclohexanecarboxylic acid (B-I) is more acidic.
Basicity:
-
Pair (C): Aniline (I) vs. o-toluidine (II)
- Aniline (I) is a primary aromatic amine.
- o-Toluidine (II) is aniline substituted with a methyl group at the ortho position.
- The methyl group is electron-donating (+I effect), which increases electron density on the nitrogen atom, making it more basic.
- However, the ortho methyl group in o-toluidine causes steric hindrance. This hindrance can impede the approach of a proton to the nitrogen atom or affect the solvation of the resulting protonated amine (conjugate acid), thereby decreasing basicity. The net effect is that o-toluidine is less basic than aniline. (pKb of aniline = 9.4, pKb of o-toluidine = 10.9).
- Therefore, aniline (C-I) is more basic.
-
Pair (D): Cyclohexylamine (I) vs. 2-methylcyclohexylamine (II)
- Cyclohexylamine (I) is a primary aliphatic amine.
- 2-methylcyclohexylamine (II) has a methyl group attached to the ring adjacent to the carbon bearing the amino group.
- The methyl group is electron-donating (+I effect). This increases electron density on the nitrogen atom, making its lone pair more available for protonation and thus increasing basicity. There is no significant steric hindrance affecting basicity in this case.
- Therefore, 2-methylcyclohexylamine (D-II) is more basic.
Combining these results: Most acidic in (A) is (II). Most acidic in (B) is (I). Most basic in (C) is (I). Most basic in (D) is (II).
This corresponds to A-II, B-I, C-I, D-II.
