Question
Question: Consider the above reaction, the major product 'P' is:...
Consider the above reaction, the major product 'P' is:
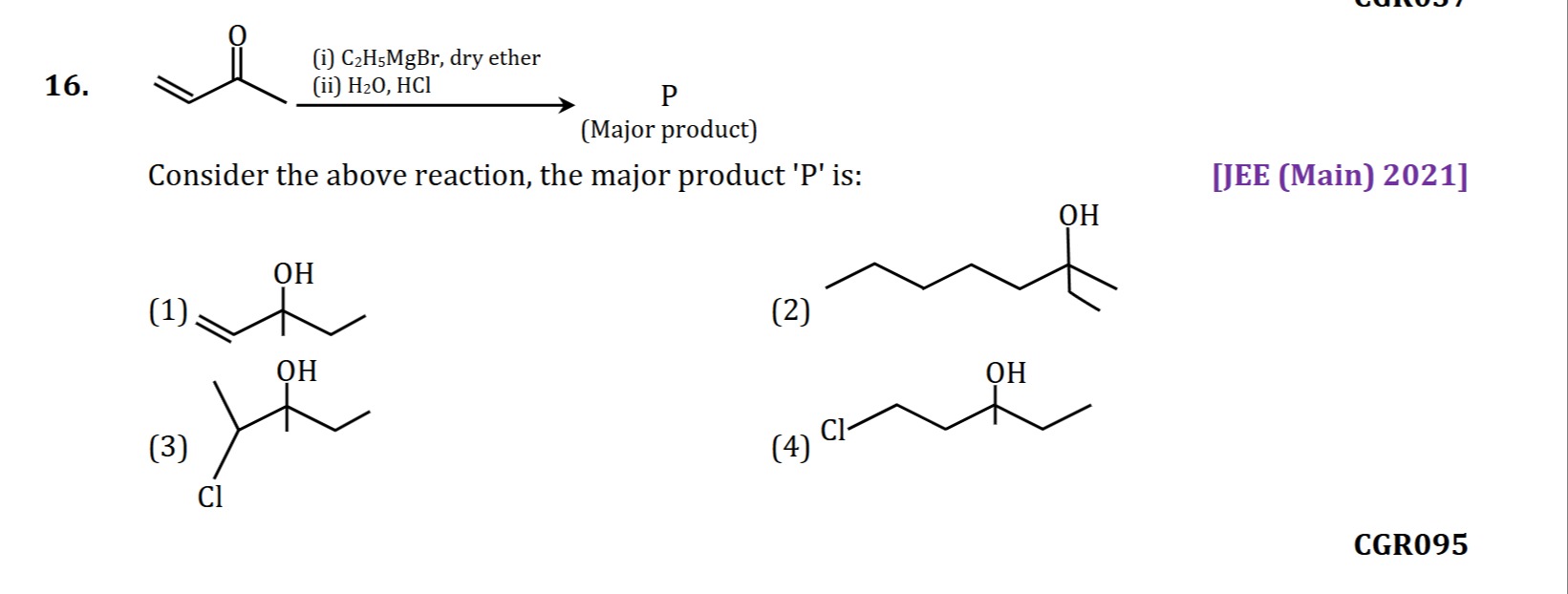
CH2=CH-C(OH)(CH3)(CH2CH3)
CH3-CH(C2H5)-CO-CH3
CH2=CH-CH(OH)-CH(CH3)2
CH2=CH-CH2-CO-CH2CH3
CH2=CH-C(OH)(CH3)(CH2CH3)
Solution
The reaction involves the addition of ethyl magnesium bromide (C2H5MgBr), a Grignard reagent, to but-3-en-2-one, followed by hydrolysis. But-3-en-2-one is an α,β-unsaturated ketone. Grignard reagents can undergo two types of addition to α,β-unsaturated carbonyl compounds: 1,2-addition (direct addition to the carbonyl carbon) and 1,4-addition (conjugate addition to the β-carbon).
The structure of but-3-en-2-one is CH2=CH−CO−CH3.
1. 1,2-Addition: The ethyl group from C2H5MgBr attacks the carbonyl carbon. CH2=CH−CO−CH3+C2H5MgBr→CH2=CH−C(OMgBr)(C2H5)−CH3 After hydrolysis with H2O,HCl: CH2=CH−C(OMgBr)(C2H5)−CH3H2O,HClCH2=CH−C(OH)(C2H5)−CH3 This product is 3-ethylpent-4-en-3-ol, which matches option (1).
2. 1,4-Addition: The ethyl group from C2H5MgBr attacks the β-carbon. CH2=CH−CO−CH3+C2H5MgBr→[CH2(C2H5)−CH=C(O−)−CH3] After hydrolysis: CH2(C2H5)−CH2−CO−CH3, which is 3-ethylpentan-3-one. This does not match any of the options.
For simple alkyl Grignard reagents reacting with α,β-unsaturated ketones, 1,2-addition is generally favored over 1,4-addition, especially at lower temperatures.
Therefore, the major product 'P' is 3-ethylpent-4-en-3-ol, which corresponds to option (1).
