Question
Question: 16. Choose the CORRECT statement(s) :-...
- Choose the CORRECT statement(s) :-
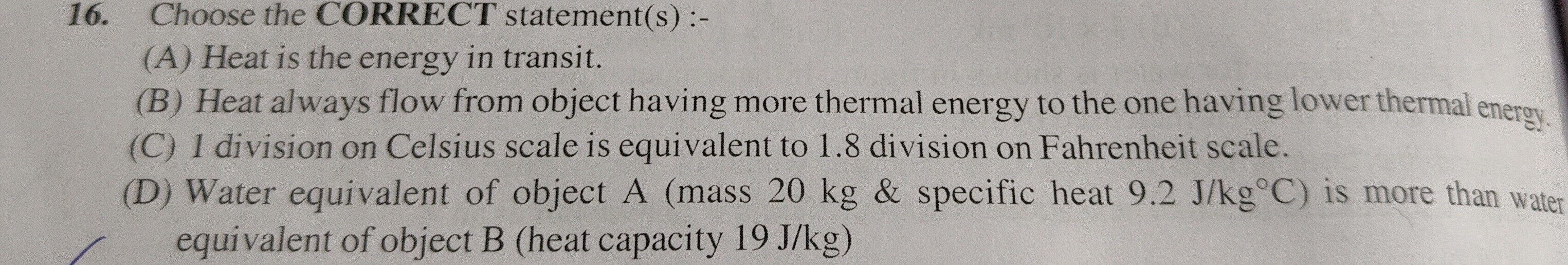
Heat is the energy in transit.
Heat always flow from object having more thermal energy to the one having lower thermal energy.
1 division on Celsius scale is equivalent to 1.8 division on Fahrenheit scale.
Water equivalent of object A (mass 20 kg & specific heat 9.2 J/kg°C) is more than water equivalent of object B (heat capacity 19 J/kg)
A, C, D
Solution
Statement (A) is correct because heat is defined as energy transferred between systems due to a temperature difference, and this energy is in transit. Statement (B) is incorrect because heat flows from higher temperature to lower temperature, not necessarily from higher thermal energy to lower thermal energy. Statement (C) is correct as the relationship ΔTF=59ΔTC implies 1 Celsius division is equivalent to 1.8 Fahrenheit divisions. For statement (D), assuming the heat capacity of object B is 19J/∘C (not J/kg), the water equivalent of A is approximately 0.044kg and that of B is approximately 0.0045kg, making WA>WB. Therefore, statements (A), (C), and (D) are correct.
