Question
Question: A cell diagram shown below contains one litre of buffer solution of HA($pK_a$ = 4) and NaA in both c...
A cell diagram shown below contains one litre of buffer solution of HA(pKa = 4) and NaA in both compartments. What is the cell e.m.f.?
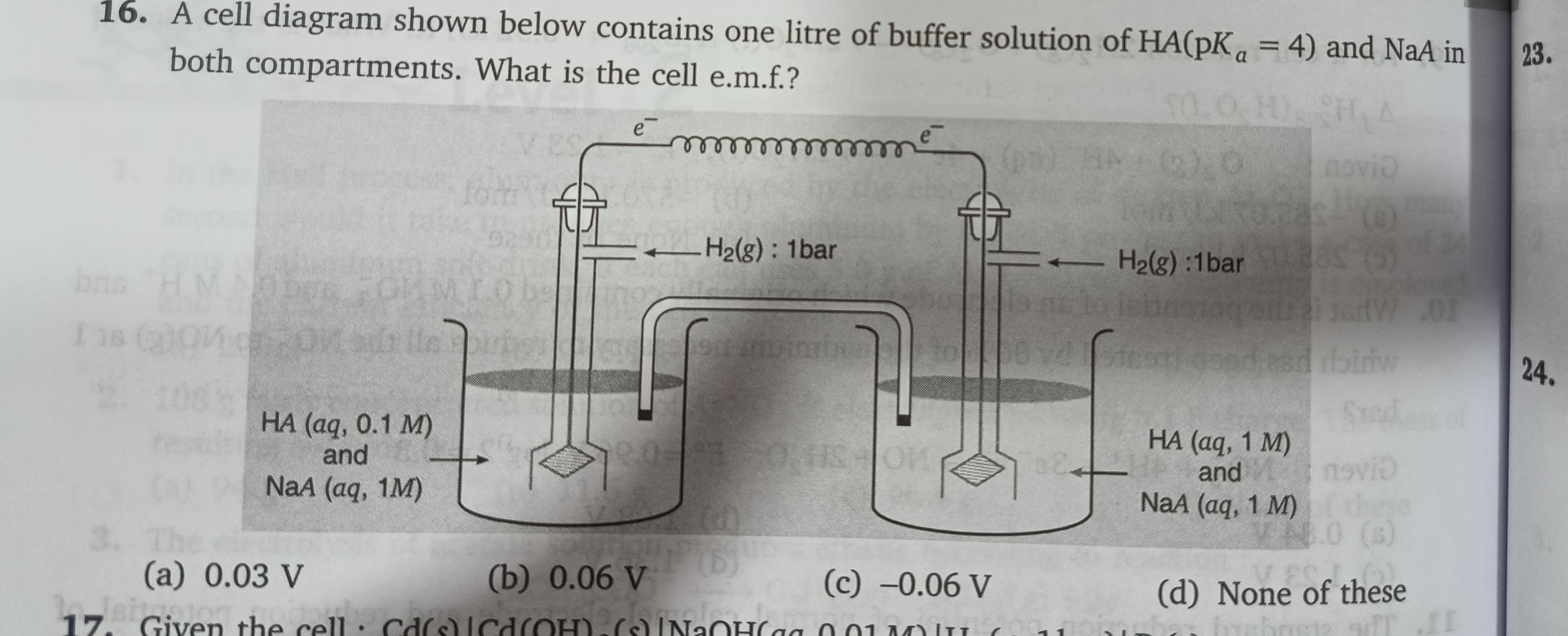
A
0.03 V
B
0.06 V
C
-0.06 V
D
None of these
Answer
0.06 V
Explanation
Solution
-
Determine pH in each half-cell using the Henderson–Hasselbalch equation:
pH=pKa+log[HA][A−]-
Left half-cell:
pH=4+log0.11=4+1=5. -
Right half-cell:
pH=4+log11=4.
-
-
Determine electrode potentials using the Nernst equation for the hydrogen electrode:
For a hydrogen electrode, the half-cell potential is given by:
E=−0.0591×pH(in volts at 298 K)-
Left electrode potential:
Eleft=−0.0591×5=−0.2955 V -
Right electrode potential:
Eright=−0.0591×4=−0.2364 V
-
-
Calculate the cell electromotive force (e.m.f.):
Ecell=Eright−Eleft=(−0.2364 V)−(−0.2955 V)=0.0591 V≈0.06 V.
Since the electrons flow from the left (more negative) electrode to the right electrode, the overall cell e.m.f. is approximately 0.06 V.
