Question
Question: In three experiments, a material A at a particular low temperature $T_c$ and a material B at a parti...
In three experiments, a material A at a particular low temperature Tc and a material B at a particular high temperature TH are placed in an isolated and insulated container. When they reach thermal equilibrium with each other (No phase change occurs), their final temperature Tf is measured. The masses mA & mB and specific heats CA & CB of the material are given in the table. Assume that heat transferred is Q in the experiment. Then which of the following is/are correct :-
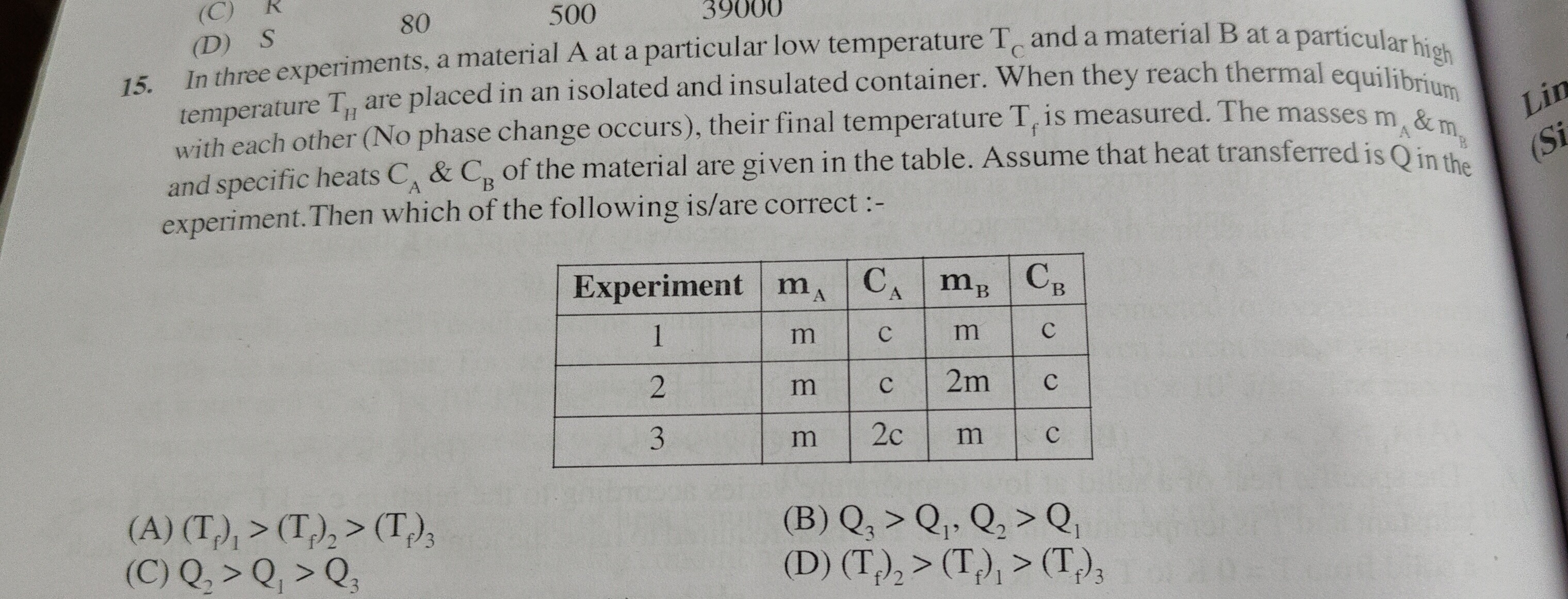
(Tf)1>(Tf)2>(Tf)3
Q3>Q1,Q2>Q1
Q2>Q1>Q3
(Tf)2>(Tf)1>(Tf)3
(B) and (D)
Solution
The principle of calorimetry states that the heat lost by the hotter substance equals the heat gained by the colder substance. The final equilibrium temperature Tf can be calculated using the formula: Tf=mACA+mBCBmACATc+mBCBTH
Experiment 1: mACA=mc, mBCB=mc. Tf1=mc+mcmc⋅Tc+mc⋅TH=2Tc+TH Q1=mc(2Tc+TH−Tc)=mc(2TH−Tc)
Experiment 2: mACA=mc, mBCB=2mc. Tf2=mc+2mcmc⋅Tc+2mc⋅TH=3Tc+2TH Q2=mc(3Tc+2TH−Tc)=mc(32TH−2Tc)=32mc(TH−Tc)
Experiment 3: mACA=2mc, mBCB=mc. Tf3=2mc+mc2mc⋅Tc+mc⋅TH=32Tc+TH Q3=2mc(32Tc+TH−Tc)=2mc(3TH−Tc)=32mc(TH−Tc)
Comparing final temperatures (assuming TH>Tc): Tf2−Tf1=6TH−Tc>0⟹Tf2>Tf1 Tf1−Tf3=6TH−Tc>0⟹Tf1>Tf3 Tf2−Tf3=3TH−Tc>0⟹Tf2>Tf3 Thus, the order of final temperatures is Tf2>Tf1>Tf3. This confirms option (D).
Comparing heat transferred, let X=mc(TH−Tc): Q1=21X Q2=32X Q3=32X Since 32>21, we have Q2=Q3>Q1. This means Q3>Q1 and Q2>Q1. This confirms option (B).
