Question
Question: Identify the products in the following reaction. Formaldehyde + Benzaldehyde i) conc. NaOH → produ...
Identify the products in the following reaction.
Formaldehyde + Benzaldehyde
i) conc. NaOH → products. ii) H3O+
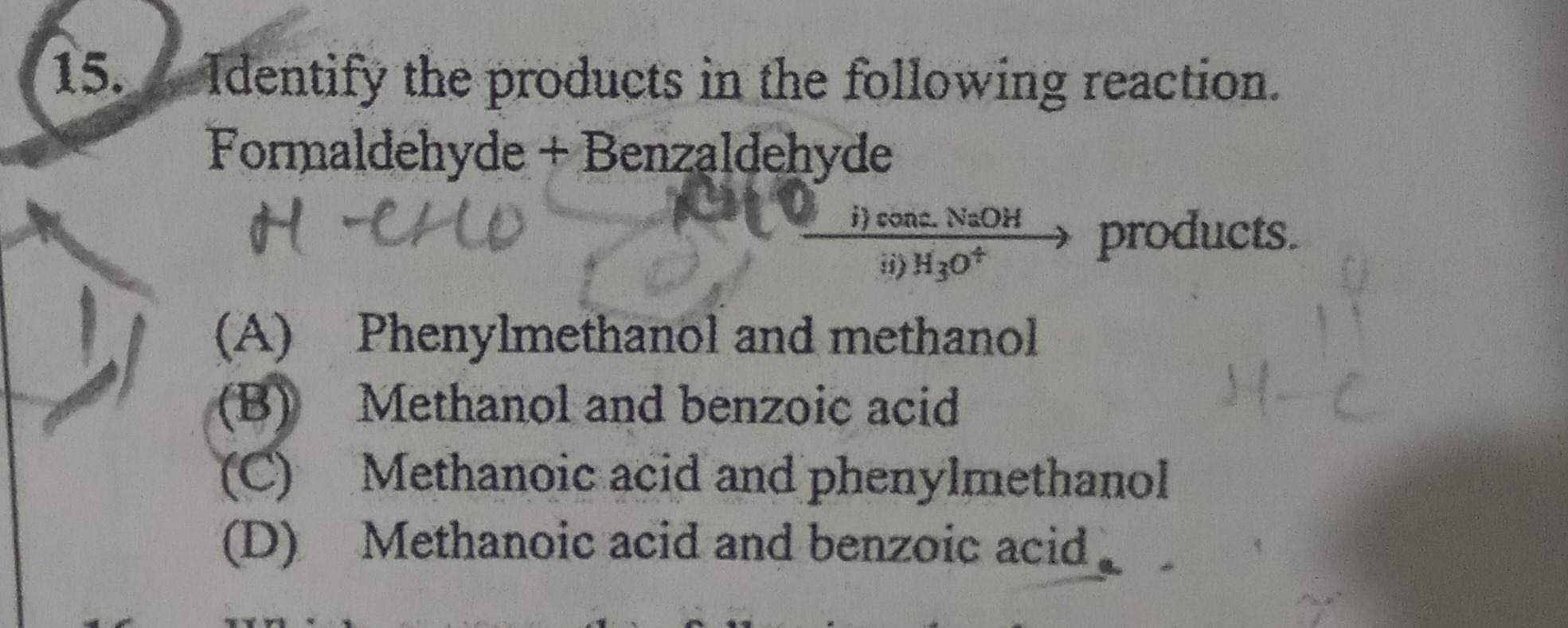
Phenylmethanol and methanol
Methanol and benzoic acid
Methanoic acid and phenylmethanol
Methanoic acid and benzoic acid
Methanol and benzoic acid
Solution
Under the given conditions (conc. NaOH followed by H₃O⁺), a Cannizzaro reaction occurs.
Both formaldehyde and benzaldehyde are non-enolizable aldehydes.
In a cross Cannizzaro reaction, the more electrophilic aldehyde (benzaldehyde) undergoes oxidation to form benzoic acid, while the less electrophilic aldehyde (formaldehyde) is reduced to form methanol.
Cannizzaro reaction of formaldehyde and benzaldehyde yields methanol (from formaldehyde via reduction) and benzoic acid (from benzaldehyde via oxidation).
