Question
Question: Consider the following six electronic configurations (remaining inner orbitals are completely filled...
Consider the following six electronic configurations (remaining inner orbitals are completely filled) and mark the incorrect option.
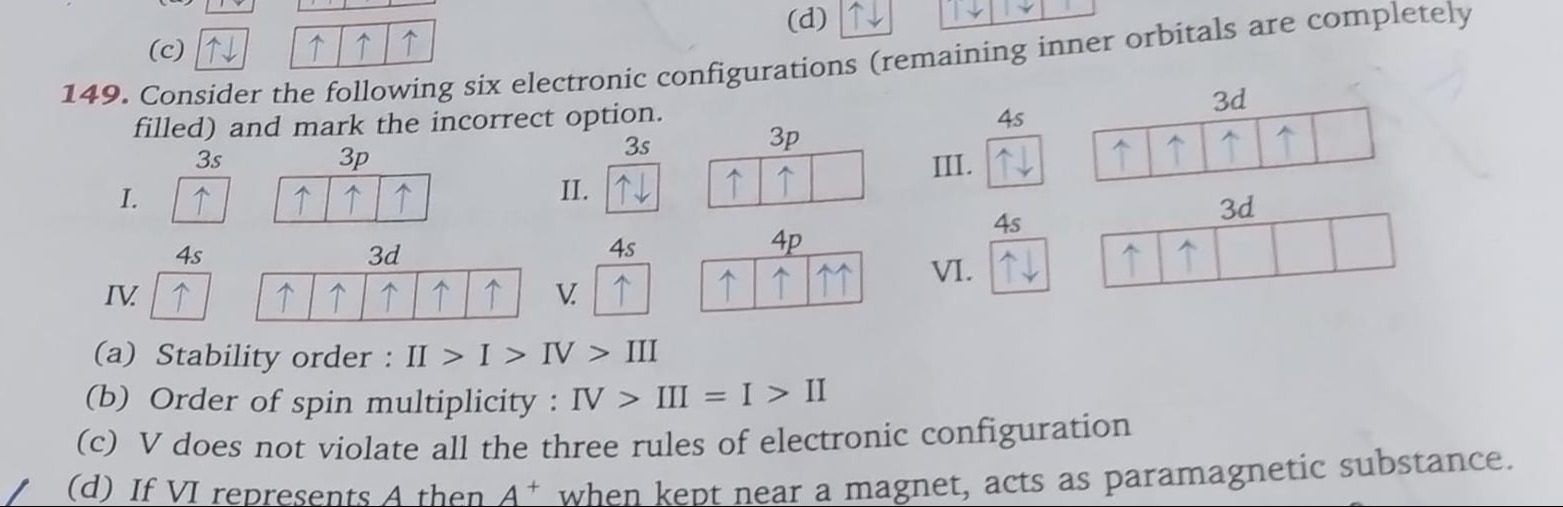
Stability order : II > I > IV > III
Order of spin multiplicity : IV > III = I > II
V does not violate all the three rules of electronic configuration
If VI represents A then A+ when kept near a magnet, acts as paramagnetic substance.
The question asks to mark the incorrect option. Based on the analysis, both options (a) and (b) are incorrect statements. Option (a) claims a stability order of II > I > IV > III, which contradicts the general principles of stability related to half-filled and fully-filled subshells. Configuration IV (4s^1 3d^5) is highly stable due to a half-filled d-subshell, while configuration II (3s^2 3p^2 3d^3) has no such stabilizing features. Option (b) claims an order of spin multiplicity of IV > III = I > II. Calculating the spin multiplicities: IV has 7, III has 5, I has 9, and II has 6. The order 7 > 5 = 9 > 6 is mathematically false. Options (c) and (d) are correct statements.
Solution
Configuration Analysis:
- I: 3s^1 3p^3 3d^4. Unpaired electrons = 1 + 3 + 4 = 8. Spin multiplicity = 2×(8/2)+1=9.
- II: 3s^2 3p^2 3d^3. Unpaired electrons = 0 + 2 + 3 = 5. Spin multiplicity = 2×(5/2)+1=6.
- III: 4s^2 3d^4. Unpaired electrons = 0 + 4 = 4. Spin multiplicity = 2×(4/2)+1=5.
- IV: 4s^1 3d^5. Unpaired electrons = 1 + 5 = 6. Spin multiplicity = 2×(6/2)+1=7.
- V: 4s^1 4p^3. Unpaired electrons = 1 + 3 = 4. Spin multiplicity = 2×(4/2)+1=5.
- VI: 4s^2 3d^2. Unpaired electrons = 0 + 2 = 2. Spin multiplicity = 2×(2/2)+1=3.
Evaluation of Options:
(a) Stability order : II > I > IV > III Stability is primarily conferred by completely filled or half-filled subshells.
- Configuration IV (4s^1 3d^5) is highly stable due to a perfectly half-filled 3d subshell.
- Configuration III (4s^2 3d^4) is relatively stable due to the filled 4s and a nearly half-filled 3d subshell.
- Configuration I (3s^1 3p^3 3d^4) has a half-filled 3p subshell.
- Configuration II (3s^2 3p^2 3d^3) lacks any significantly stabilizing filled or half-filled subshells in its valence shell.
The order II > I > IV > III is incorrect as configuration IV is expected to be the most stable among these.
(b) Order of spin multiplicity : IV > III = I > II Using the calculated spin multiplicities: IV=7, III=5, I=9, II=6. The statement translates to: 7>5=9>6. This is mathematically incorrect.
(c) V does not violate all the three rules of electronic configuration Configuration V is 4s^1 4p^3.
- Aufbau principle: 4s is filled before 4p. Satisfied.
- Pauli exclusion principle: No orbital contains more than two electrons with opposite spins. Satisfied.
- Hund's rule: Electrons are distributed singly in degenerate orbitals with parallel spins before pairing. The three electrons in 4p are placed in separate orbitals with parallel spins. Satisfied. Since configuration V follows all three rules, the statement "V does not violate all the three rules" is true.
(d) If VI represents A then A+ when kept near a magnet, acts as paramagnetic substance. Configuration VI (4s^2 3d^2) represents Titanium (Ti, Z=22). So, A = Ti. When Ti loses an electron to form A+ (Ti+), the electron is removed from the outermost shell (4s). Ti+ has the configuration 4s^1 3d^2. In Ti+, there is 1 unpaired electron in the 4s orbital and 2 unpaired electrons in the 3d orbitals. The presence of unpaired electrons makes the substance paramagnetic. Thus, the statement (d) is true.
Since the question asks to mark the incorrect option, and both (a) and (b) are incorrect statements, they are the answers.
