Question
Question: The following reaction has an equilibrium constant $K_C$ equal to 3.07 x $10^{-4}$ at 24°C. $2NOBr(g...
The following reaction has an equilibrium constant KC equal to 3.07 x 10−4 at 24°C. 2NOBr(g)⇌2NO(g)+Br2(g)
The correct set of concentrations at which the rate of forward reaction is greater than that of backward reaction is
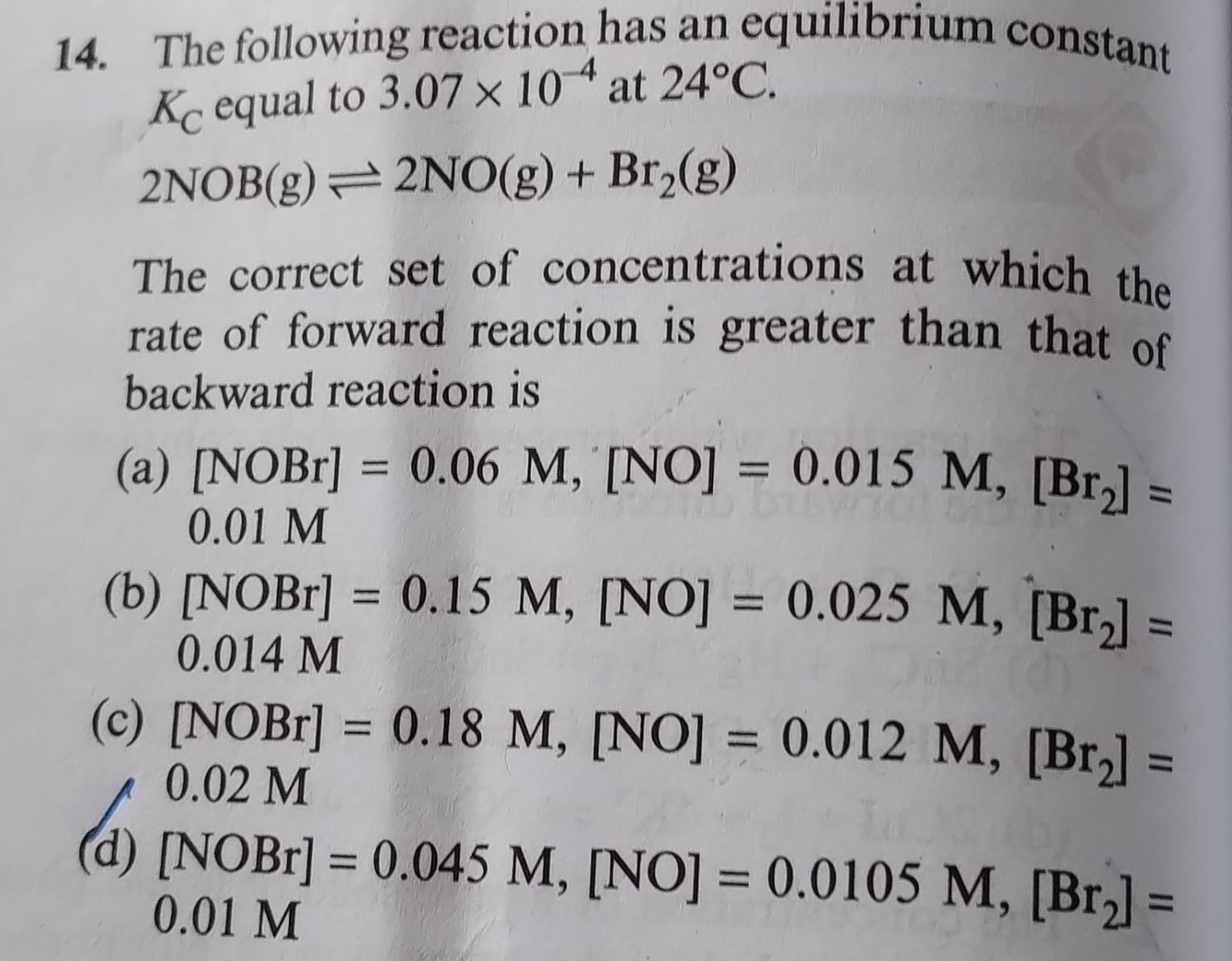
[NOBr] = 0.06 M, [NO] = 0.015 M, [Br2] = 0.01 M
[NOBr] = 0.15 M, [NO] = 0.025 M, [Br2] = 0.014 M
[NOBr] = 0.18 M, [NO] = 0.012 M, [Br2] = 0.02 M
[NOBr] = 0.045 M, [NO] = 0.0105 M, [Br2] = 0.01 M
[NOBr] = 0.18 M, [NO] = 0.012 M, [Br2] = 0.02 M
Solution
The rate of the forward reaction is greater than the backward reaction when the reaction quotient (QC) is less than the equilibrium constant (KC). For the reaction 2NOBr(g)⇌2NO(g)+Br2(g), the reaction quotient is QC=[NOBr]2[NO]2[Br2]. Given KC=3.07×10−4. Calculating QC for option (c): QC=(0.18)2(0.012)2×0.02=3.24×10−2(1.44×10−4)×(2×10−2)=3.24×10−22.88×10−6≈8.89×10−5. Since 8.89×10−5<3.07×10−4, QC<KC, indicating the forward reaction rate is greater.
