Question
Question: The E° in the given diagram is :- ...
The E° in the given diagram is :-
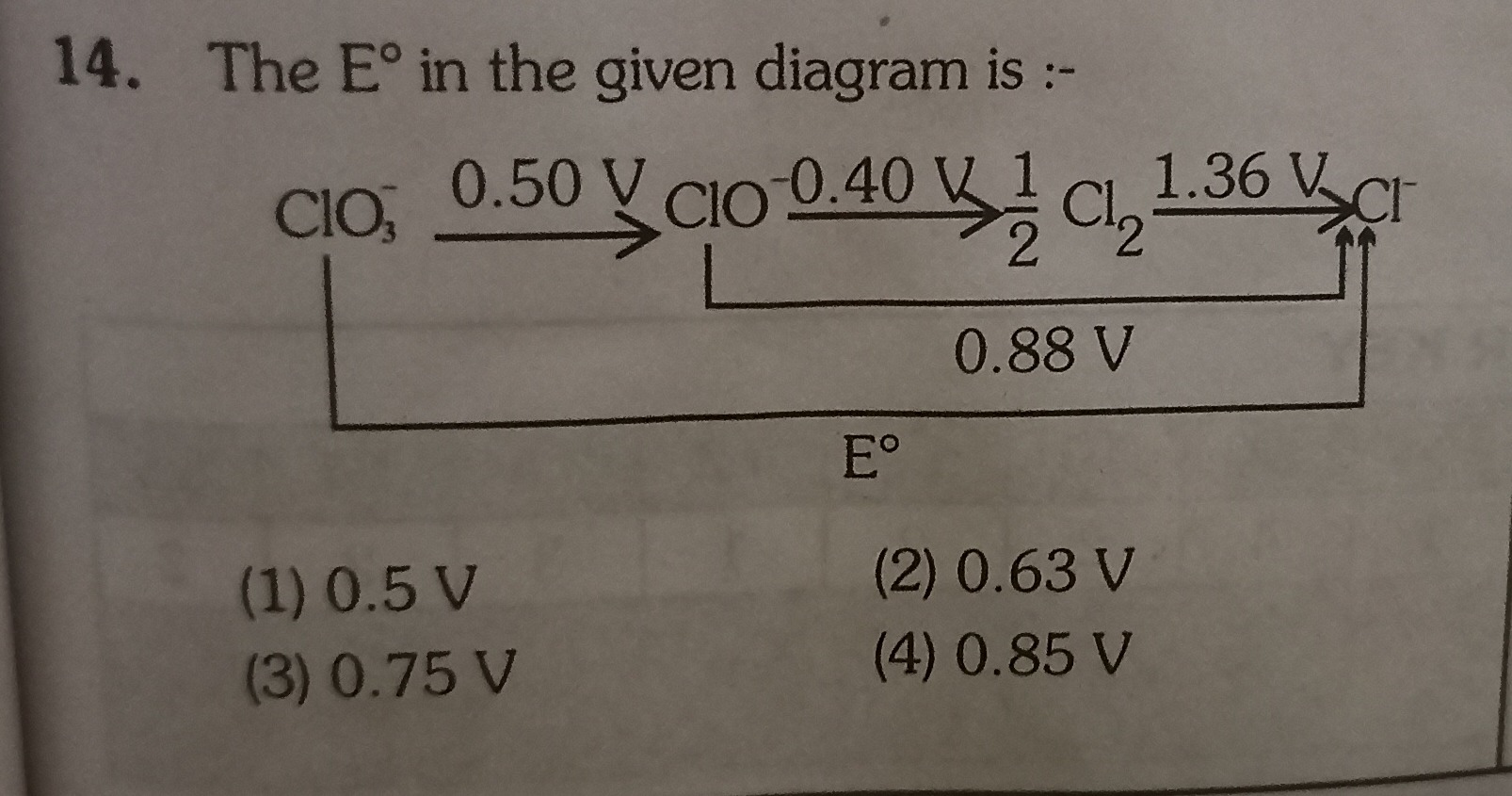
0.5 V
0.63 V
0.75 V
0.85 V
0.85 V
Solution
The question asks for the value of E° marked in the Latimer diagram, which corresponds to the standard reduction potential for the conversion of ClO− to Cl−. This overall reduction occurs in two steps:
-
ClO−→21Cl2: This step involves a change in oxidation state from +1 to 0, meaning 1 electron (n1=1) is transferred. The given E1∘=0.40V.
-
21Cl2→Cl−: This step involves a change in oxidation state from 0 to -1, meaning 1 electron (n2=1) is transferred. The given E2∘=1.36V.
To find the overall standard potential (E°) for ClO−→Cl−, we use the additivity of Gibbs free energy (ΔG∘=−nFE∘).
The total number of electrons transferred for the overall reaction is ntotal=n1+n2=1+1=2.
The total Gibbs free energy change is the sum of the individual steps' Gibbs free energy changes:
ΔGtotal∘=ΔG1∘+ΔG2∘=(−n1FE1∘)+(−n2FE2∘)
ΔGtotal∘=−(1×F×0.40)+−(1×F×1.36)
ΔGtotal∘=−0.40F−1.36F=−1.76F
Now, relate the total Gibbs free energy change to the overall standard potential E°:
ΔGtotal∘=−ntotalFE∘
−1.76F=−2FE∘
E∘=21.76=0.88V
The calculated value is 0.88 V. Among the given options, 0.85 V is the closest.
