Question
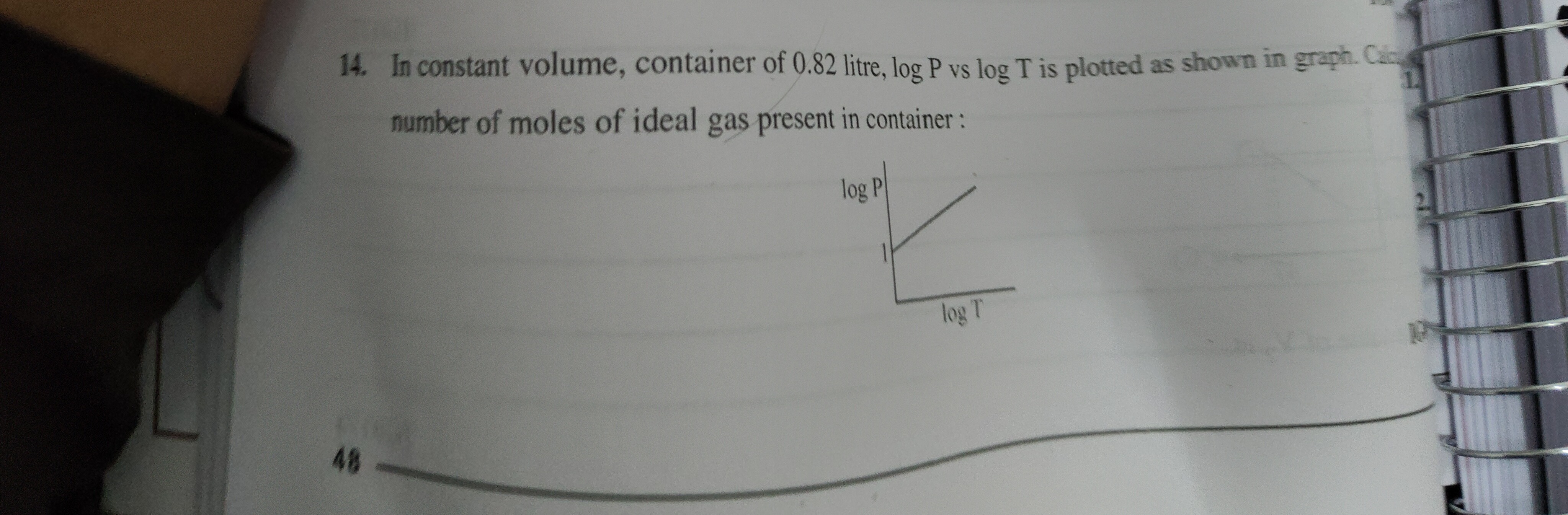
Question: In constant volume, container of 0.82 litre, log P vs log T is plotted as shown in graph. Calc numbe...
In constant volume, container of 0.82 litre, log P vs log T is plotted as shown in graph. Calc number of moles of ideal gas present in container:
Answer
100
Explanation
Solution
The ideal gas law is PV=nRT. Since the volume (V) is constant, we can rearrange the equation to P=VnRT. Taking the logarithm of both sides, we get logP=log(VnR)+logT. This equation is in the form of a straight line y=mx+c, where y=logP, x=logT, the slope m=1, and the y-intercept c=log(VnR). From the graph, the y-intercept is given as 1. Therefore, log(VnR)=1, which implies VnR=101=10. Given V=0.82 litre and using the ideal gas constant R=0.0821 L atm/(mol K), we can solve for the number of moles (n): n=R10×V=0.0821 L atm / (mol K)10×0.82 L=0.08218.2 mol=100 mol.
