Question
Question: H₂ gas is kept inside a container A and container B each having volume 2 litre under different c whi...
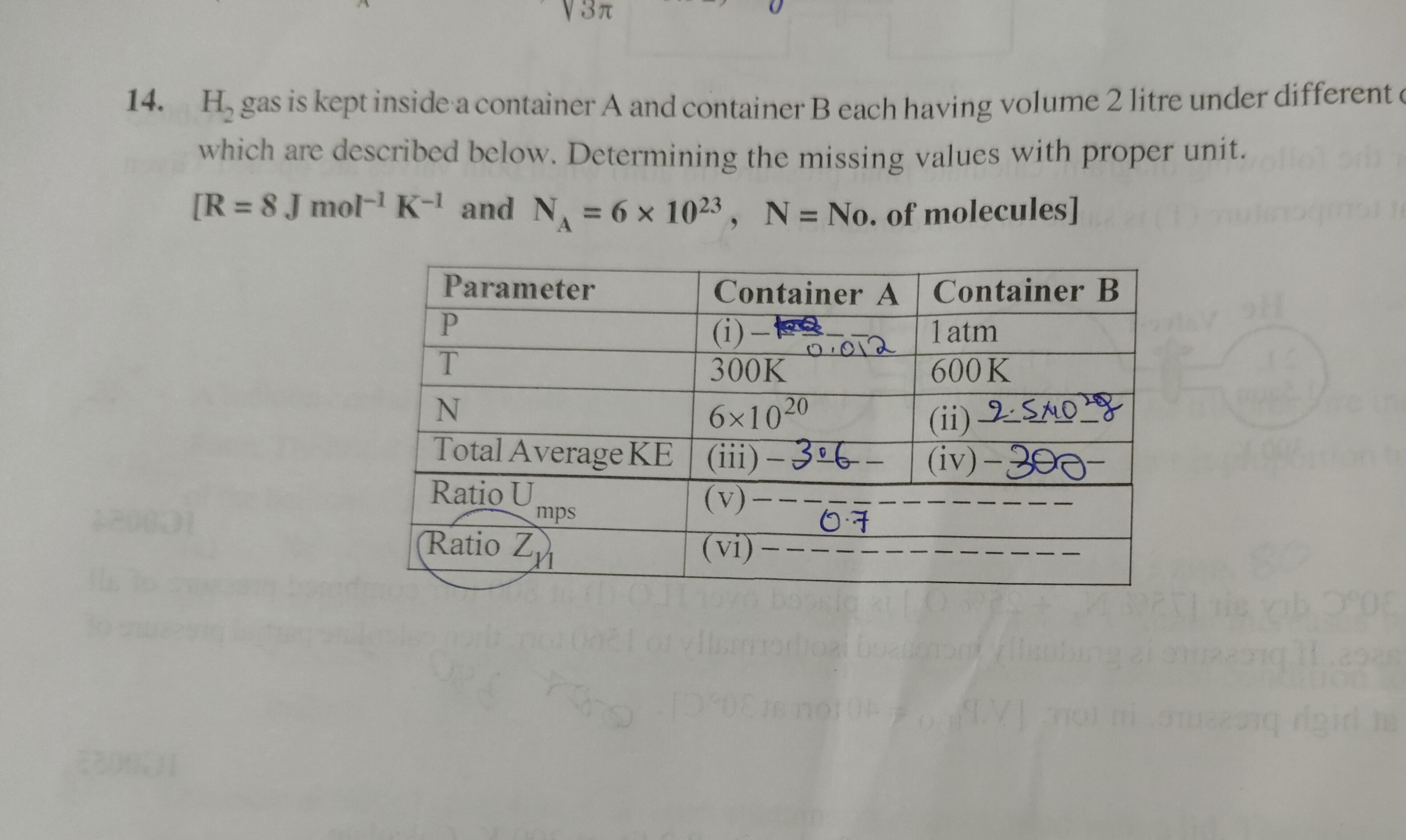
H₂ gas is kept inside a container A and container B each having volume 2 litre under different c which are described below. Determining the missing values with proper unit. [R = 8 J mol⁻¹ K⁻¹ and Nᴀ = 6 x 10²³, N = No. of molecules]
Answer
(i) 1200Pa, (ii) 2.533×1022, (iii) 3600J, (iv) 303.975J, (v) 1897.37m/s, (vi) 1549.19m/s
Explanation
Solution
- Calculated moles for Container A (nA) from given molecules (NA) and Avogadro's number (NA): nA=6×10236×1020=1×10−3mol.
- Used Ideal Gas Law (PV=nRT) to find Pressure for Container A (PA): PA=VnARTA=2×10−3(1×10−3)×8×300=1200Pa. This is (i).
- Calculated Total Average Kinetic Energy for Container A (KEavg,A) using KEavg=23nRT: KEavg,A=23×(1×10−3)×8×300=3600J. This is (iii).
- Calculated Root Mean Square Speed for Container A (Urms,A) using Urms=M3RT (Molar mass of H₂ = 2 g/mol = 2×10−3 kg/mol): Urms,A=2×10−33×8×300=3.6×106≈1897.37m/s. This is (v).
- Calculated Most Probable Speed for Container A (Z1,A) using Z1=M2RT: Z1,A=2×10−32×8×300=2.4×106≈1549.19m/s. This is (vi).
- Calculated moles for Container B (nB) from given pressure (PB=1atm=101325Pa), volume (V=2×10−3m3), and temperature (TB=600K) using Ideal Gas Law: nB=RTBPBV=8×600101325×2×10−3≈0.0422mol.
- Calculated Number of Molecules for Container B (NB) from moles (nB) and Avogadro's number (NA): NB=nB×NA=0.0422×6×1023≈2.533×1022. This is (ii).
- Calculated Total Average Kinetic Energy for Container B (KEavg,B) using KEavg=23nRT: KEavg,B=23×0.0422×8×600≈303.975J. This is (iv).
- The value "0.7" in the table for Container B under "Ratio U_rms" is interpreted as the ratio Urms,BUrms,A, as TBTA=600300=0.5≈0.707. The direct calculation of Urms,B would be 2×10−33×8×600≈2683.3m/s, which is not represented by 0.7.
