Question
Question: Which one of the following is most stable ?...
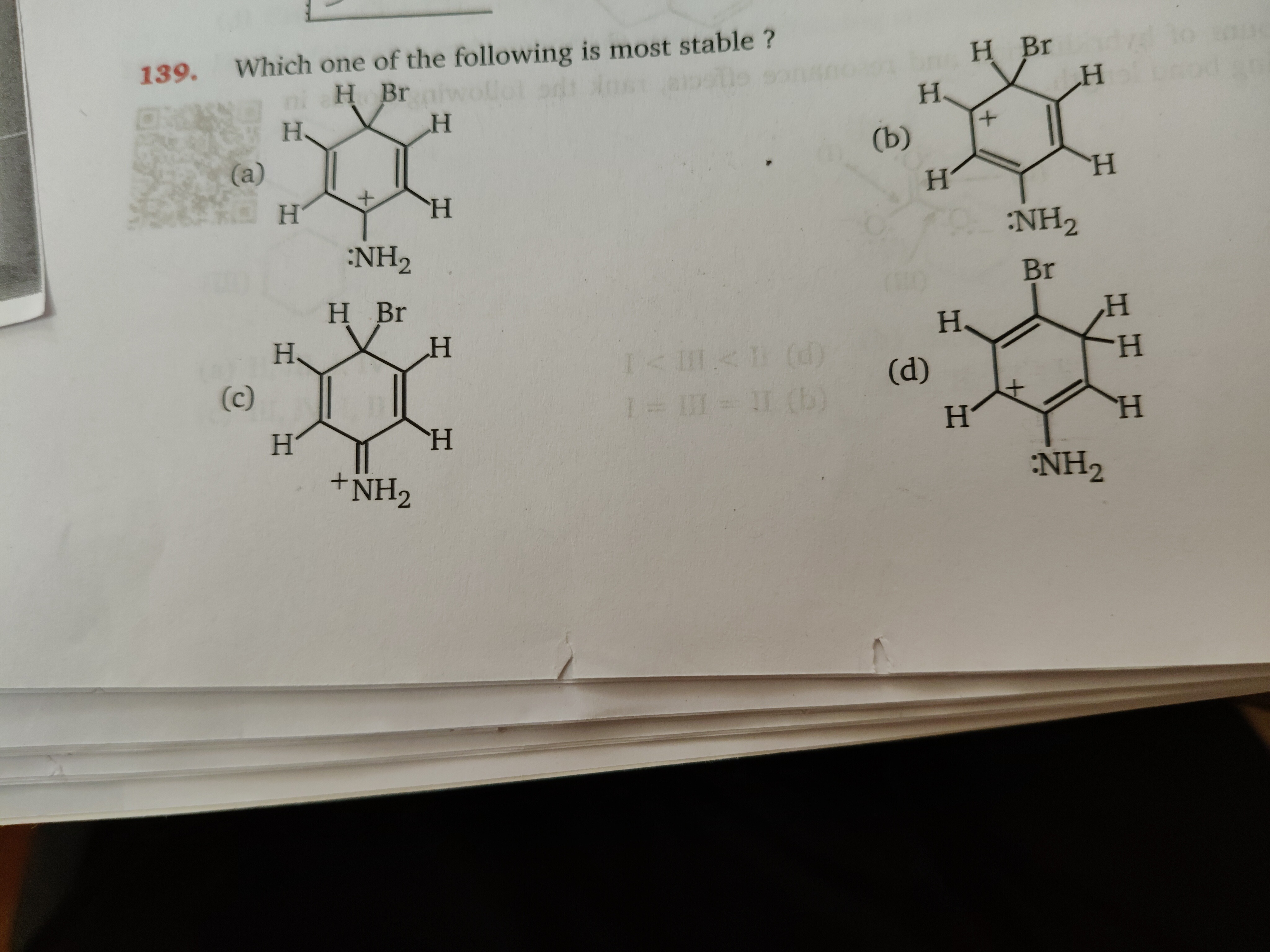
Which one of the following is most stable ?
\begin{array}{c} \text{H} \quad \text{Br}\\ \text{H}\text{-}\begin{smallmatrix} \diagup \diagdown \\ \diagdown \diagup \end{smallmatrix}\text{-H}\\ \text{H} \quad \text{NH}_2 \end{array}
\begin{array}{c} \text{H} \quad \text{Br}\\ \text{H}\text{-}\begin{smallmatrix} \diagup \diagdown \\ \diagdown \diagup \end{smallmatrix}\text{-H}\\ \text{H} \quad \text{NH}_2 \end{array}
\begin{array}{c} \text{H} \quad \text{Br}\\ \text{H}\text{-}\begin{smallmatrix} \diagup \diagdown \\ \diagdown \diagup \end{smallmatrix}\text{-H}\\ \text{H} \quad \text{NH}_2 \end{array}
\begin{array}{c} \text{H} \quad \text{Br}\\ \text{H}\text{-}\begin{smallmatrix} \diagup \diagdown \\ \diagdown \diagup \end{smallmatrix}\text{-H}\\ \text{H} \quad \text{NH}_2 \end{array}
\begin{array}{c} \text{H} \quad \text{Br}\\ \text{H}\text{-}\begin{smallmatrix} \diagup \diagdown \\ \diagdown \diagup \end{smallmatrix}\text{-H}\\ \text{H} \quad \text{NH}_2 \end{array}
Solution
The stability of carbocations is influenced by resonance, inductive effects, and hyperconjugation. Electron-donating groups enhance stability, while electron-withdrawing groups decrease it. Resonance delocalization of a positive charge significantly increases stability.
Let's analyze each structure in terms of stability:
-
Structure (a): This is a cyclohexadienyl cation with a bromine (Br) atom at the ortho position and an amino (:NH₂) group at the para position relative to the positive charge. The NH₂ group is a strong electron-donating group via resonance (+M effect), which effectively stabilizes the carbocation when the positive charge is delocalized to the para position. The Br atom, being electron-withdrawing via induction (-I effect), destabilizes the carbocation, particularly at the ortho position.
-
Structure (b): This cyclohexadienyl cation has the NH₂ group at the meta position and the Br atom also at the meta position relative to the positive charge. The +M effect of NH₂ is not directly effective at the meta position for stabilizing the positive charge. The -I effect of Br is also less pronounced at the meta position compared to the ortho position.
-
Structure (c): This structure depicts an iminium ion, where the nitrogen atom of the amino group bears a positive charge and forms a double bond with the carbon atom. This is a resonance structure of the species in option (a). It arises when the positive charge is delocalized onto the carbon bearing the NH₂ group, and the NH₂ group donates its lone pair to form a C=N⁺ double bond.
-
Structure (d): This is a cyclohexadienyl cation with the NH₂ group at the para position and the Br atom at the meta position relative to the positive charge. Similar to (a), the para-NH₂ group provides strong resonance stabilization. However, in this case, the Br atom is at the meta position, meaning its destabilizing inductive effect is weaker than when it is at the ortho position (as in (a)).
Comparison of Stability:
- Resonance Stabilization: Structures (a) and (d) benefit from the strong resonance donation of the para-NH₂ group, which is the most significant stabilizing factor. Structure (b), with NH₂ at the meta position, receives less resonance stabilization.
- Inductive Effects: Bromine is electron-withdrawing and destabilizes carbocations.
- In (a), Br is ortho to the positive charge, exerting a strong -I effect.
- In (d), Br is meta to the positive charge, exerting a weaker -I effect.
- In (b), Br is meta to the positive charge.
Comparing (a) and (d): Both have the highly stabilizing para-NH₂ group. However, structure (d) is more stable than (a) because the destabilizing inductive effect of the Br atom is at a meta position (weaker) in (d) compared to the ortho position (stronger) in (a).
Structure (c) is a resonance contributor to the species in (a). The overall stability of a species is a weighted average of its resonance contributors. While structure (c) involves a C=N⁺ double bond, the positive charge is on a more electronegative atom (N) compared to carbon, which is generally less favorable. The iminium ion form is crucial for understanding the resonance of the species in (a). When comparing individual resonance structures, the one with the positive charge on carbon (like in (d)) is generally considered more stable than the iminium ion form (c), especially when the carbon bearing the positive charge is stabilized by electron-donating groups.
Based on this analysis, structure (d) is the most stable among the given options.
