Question
Question: What is the correct order of acidity of the protons marked A-D in the given compounds ?...
What is the correct order of acidity of the protons marked A-D in the given compounds ?
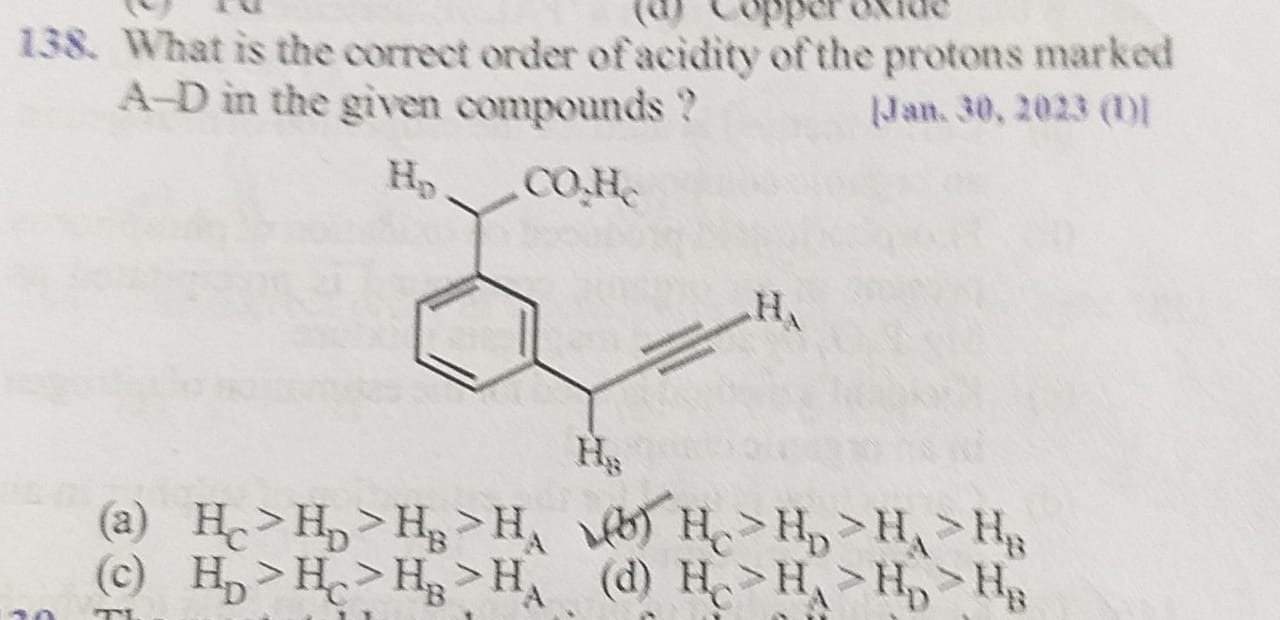
A
HC>HD>HB>HA
B
HC>HD>HA>HB
C
HD>HC>HB>HA
D
HC>HA>HD>HB
Answer
HC>HD>HA>HB
Explanation
Solution
The acidity of a proton is determined by the stability of the conjugate base formed upon its removal.
- HC is a carboxylic acid proton, forming a resonance-stabilized carboxylate anion.
- HD is alpha to a carbonyl and benzylic, stabilized by resonance with the phenyl ring and inductive effect of the carbonyl.
- HA is a terminal alkyne proton, forming a resonance-stabilized acetylide anion due to the sp hybridization of the carbon.
- HB is benzylic and alpha to an alkyne, stabilized by resonance with the phenyl ring.
Comparing the factors: Carboxylate resonance > alpha-carbonyl/benzylic stabilization > sp hybridization > benzylic/alkyne stabilization. Thus, the order of acidity is HC>HD>HA>HB.
