Question
Question: Which one of the following is not a base?...
Which one of the following is not a base?
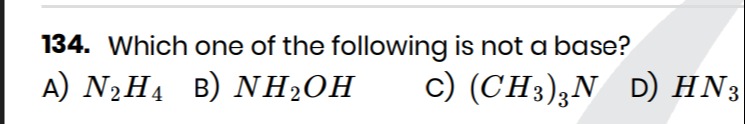
A
N2H4
B
NH2OH
C
(CH3)3N
D
HN3
Answer
D) HN3
Explanation
Solution
Hydrazine (N2H4), hydroxylamine (NH2OH), and trimethylamine ((CH3)3N) are all bases because their nitrogen atoms possess lone pairs of electrons, enabling them to accept protons (Brønsted-Lowry bases) or donate electron pairs (Lewis bases). Hydrazoic acid (HN3), however, is an acid, characterized by its ability to donate a proton. Thus, HN3 is not a base.
