Question
Question: Which of the following group show -M effect?...
Which of the following group show -M effect?
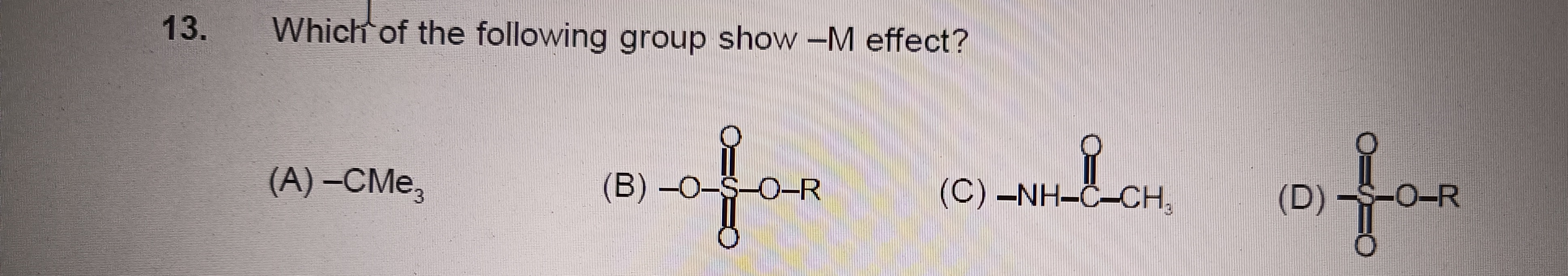
-CMe3
-O-SO₂-OR
-NH-C(=O)-CH₃
-SO₂-OR
D
Solution
-M effect is shown by groups that withdraw electrons from a conjugated system via resonance. This typically occurs when the atom directly attached to the conjugated system is part of a multiple bond with a more electronegative atom, or has vacant d-orbitals to accept electrons.
(A) -CMe₃ is an alkyl group, showing +I effect.
(B) -O-SO₂-OR is attached via oxygen, which has lone pairs, thus showing +M effect.
(C) -NH-C(=O)-CH₃ is attached via nitrogen, which has a lone pair, thus showing +M effect.
(D) -SO₂-OR is attached via sulfur, which is double-bonded to electronegative oxygen atoms and has empty d-orbitals. It can accept electrons from the conjugated system, hence showing a -M effect.
