Question
Question: Which compound has non-zero dipole moment?...
Which compound has non-zero dipole moment?
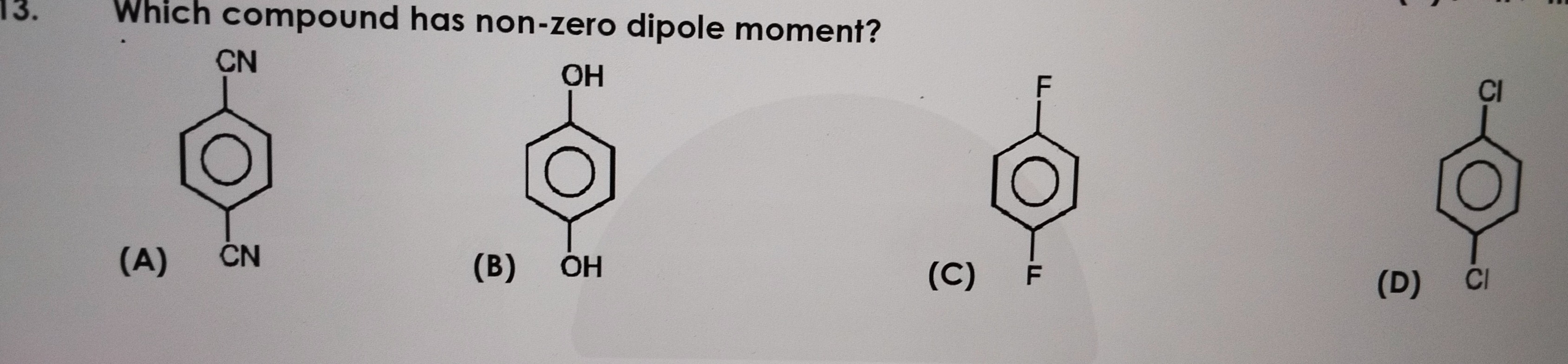
The compound with a non-zero dipole moment is (B).
Solution
The dipole moment of a molecule is the vector sum of the individual bond dipoles. For a molecule to have a zero dipole moment, the vector sum of all bond dipoles must be zero. This often occurs in molecules with high symmetry, where individual bond dipoles cancel each other out.
Let's analyze each option:
A) para-dicyanobenzene: The structure is a benzene ring with two -CN groups at para positions. The C-CN bond is polar, and the dipole moment points from the carbon of the ring towards the nitrogen of the cyano group. Since the two -CN groups are identical and are located directly opposite to each other (para position), their individual bond dipoles are equal in magnitude and opposite in direction. Thus, they cancel each other out. Therefore, para-dicyanobenzene has a net dipole moment of zero.
The dipole vectors for CN1 and CN2 are equal and opposite, leading to cancellation.
B) para-dihydroxybenzene (Hydroquinone): The structure is a benzene ring with two -OH groups at para positions. The -OH group is polar due to the electronegativity difference between oxygen and hydrogen (O-H bond) and carbon and oxygen (C-O bond). More importantly, the oxygen atom in the -OH group is sp3 hybridized, and the C-O-H angle is bent (approximately 109.5°). This means the dipole moment of the -OH group is not directed along the C-O bond axis. Due to the bent geometry and the presence of lone pairs on oxygen, the individual dipole moments of the two -OH groups, even though they are in para positions, do not perfectly cancel each other out through vector addition. There is also free rotation around the C-O bond, allowing for various conformations, most of which contribute to a net dipole moment. Therefore, para-dihydroxybenzene has a non-zero dipole moment.
The dipole vectors for the -OH groups are not directly opposite due to the bent geometry, leading to a non-zero net dipole.
C) para-difluorobenzene: The structure is a benzene ring with two -F atoms at para positions. The C-F bond is highly polar, with the dipole pointing from carbon towards fluorine. Similar to option (A), the two identical fluorine atoms are directly opposite to each other. Their individual C-F bond dipoles are equal in magnitude and opposite in direction, leading to cancellation. Therefore, para-difluorobenzene has a net dipole moment of zero.
D) para-dichlorobenzene: The structure is a benzene ring with two -Cl atoms at para positions. The C-Cl bond is polar, with the dipole pointing from carbon towards chlorine. Again, the two identical chlorine atoms are directly opposite to each other. Their individual C-Cl bond dipoles are equal in magnitude and opposite in direction, leading to cancellation. Therefore, para-dichlorobenzene has a net dipole moment of zero.
Conclusion: Compounds (A), (C), and (D) are para-disubstituted benzenes with identical substituents that are either linear (like -CN) or spherically symmetric (like -F, -Cl). In such cases, the bond dipoles cancel out due to symmetry. Compound (B), para-dihydroxybenzene, has -OH groups which are non-linear due to the bent geometry around the oxygen atom and the presence of lone pairs. The individual dipole moment of an -OH group is not aligned along the C-O bond axis. Consequently, even in a para arrangement, the vector sum of these group dipoles does not cancel out completely, resulting in a non-zero dipole moment for the molecule.
The final answer is B.
Explanation of the solution: Dipole moment is the vector sum of bond dipoles. (A) para-dicyanobenzene: Two identical, linear -CN groups are para. Their bond dipoles are equal and opposite, cancelling out. Net dipole = 0. (B) para-dihydroxybenzene (Hydroquinone): Two identical -OH groups are para. The -OH group has a bent geometry around oxygen (C-O-H angle is not 180°), meaning its group dipole is not aligned along the C-O axis. Due to this non-linearity and conformational flexibility, the vector sum of the two -OH group dipoles does not cancel out. Net dipole ≠ 0. (C) para-difluorobenzene: Two identical, spherically symmetric -F atoms are para. Their bond dipoles are equal and opposite, cancelling out. Net dipole = 0. (D) para-dichlorobenzene: Two identical, spherically symmetric -Cl atoms are para. Their bond dipoles are equal and opposite, cancelling out. Net dipole = 0. Therefore, only para-dihydroxybenzene has a non-zero dipole moment.
