Question
Question: The major products A and B for the following reactions are, respectively:...
The major products A and B for the following reactions are, respectively:
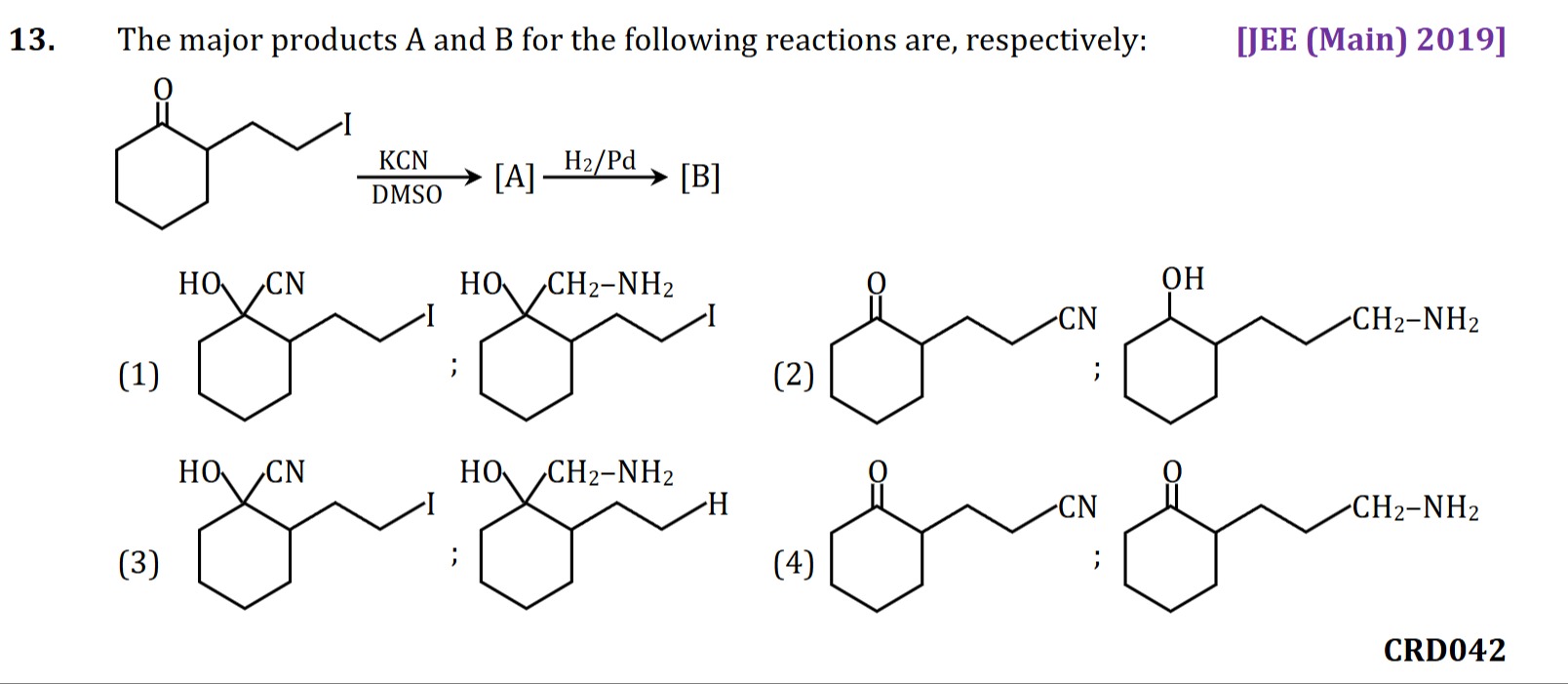
2
Solution
The reaction sequence begins with 2-(3-iodopropyl)cyclohexanone.
Step 1: Reaction with KCN in DMSO. KCN provides the nucleophilic cyanide ion (CN−), and DMSO is a polar aprotic solvent favoring SN2 reactions. The cyanide ion, being a soft nucleophile, preferentially attacks the primary alkyl iodide via an SN2 mechanism, displacing the iodide ion. This yields Product A, which is 2-(3-cyanopropyl)cyclohexanone. Nucleophilic addition to the carbonyl carbon (forming a cyanohydrin) is less favored due to the carbonyl carbon being a harder electrophile compared to the primary alkyl halide.
Step 2: Reaction with H2/Pd. Product A is then treated with H2/Pd, a catalyst for hydrogenation. This reagent reduces nitrile groups (-CN) to primary amines (-CH2NH2) and ketone groups (C=O) to secondary alcohols (-CHOH). In this case, the nitrile group is reduced to a primary amine, forming Product B, which is 2-(3-aminopropyl)cyclohexanone. The options suggest that the ketone group remains unreacted under these specific conditions, indicating selective reduction of the nitrile.
Therefore, Product A is 2-(3-cyanopropyl)cyclohexanone and Product B is 2-(3-aminopropyl)cyclohexanone, corresponding to option (2).
