Question
Question: Calculate $\Delta G^{\circ}$ for the following cell reaction $Zn(s) + Ag_2O(s) + H_2O(l) \rightarro...
Calculate ΔG∘ for the following cell reaction
Zn(s)+Ag2O(s)+H2O(l)→Zn2+(aq)+2Ag(s)+2OH−(aq)
EAg+/Ag0=+0.80V and EZn+2/Zn0=−0.76V,F=96500
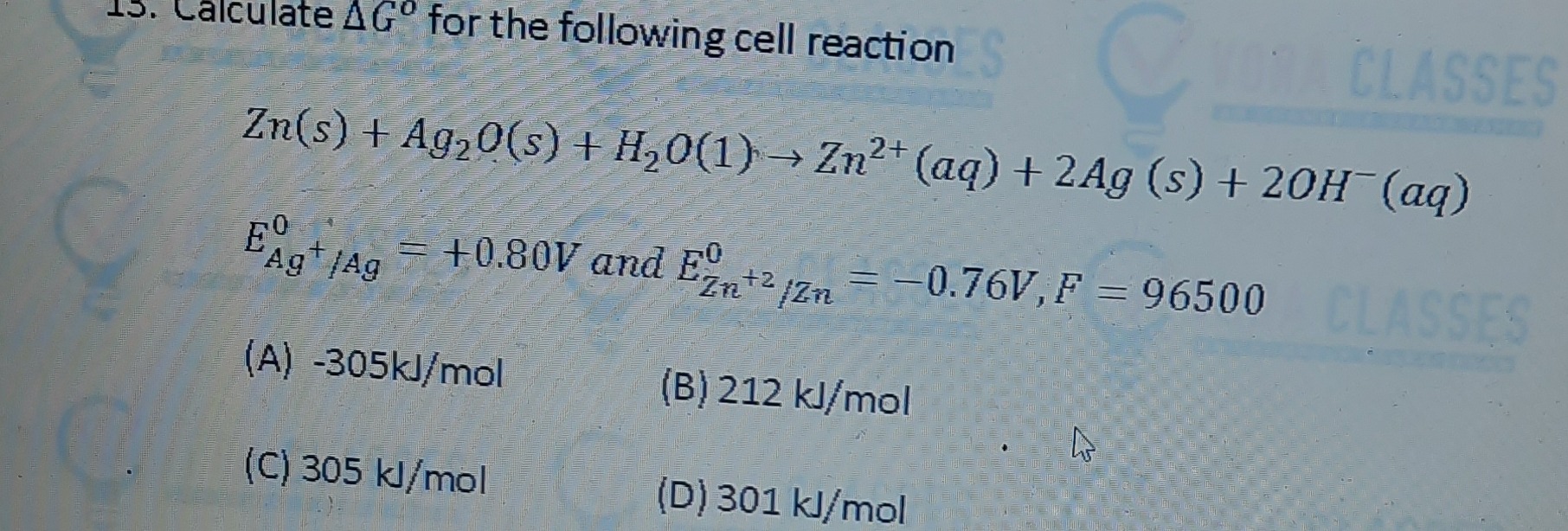
-305kJ/mol
212 kJ/mol
305 kJ/mol
301 kJ/mol
-305kJ/mol
Solution
To calculate ΔG∘, we use the formula:
ΔG∘=−nFEcell∘
First, we need to identify the half-reactions and the number of electrons (n) transferred.
- Oxidation half-reaction (Anode):
Zn(s)→Zn2+(aq)+2e−
- Reduction half-reaction (Cathode):
Ag2O(s)+H2O(l)+2e−→2Ag(s)+2OH−(aq)
From the balanced half-reactions, it is clear that n=2 electrons are transferred in the overall reaction.
Next, we calculate the standard cell potential (Ecell∘). The standard electrode potentials given are:
EAg+/Ag0=+0.80V (Standard reduction potential for the cathode)
EZn2+/Zn0=−0.76V (Standard reduction potential for the anode)
The standard cell potential is calculated as:
Ecell∘=Ecathode∘−Eanode∘
Ecell∘=EAg+/Ag0−EZn2+/Zn0
Ecell∘=(+0.80V)−(−0.76V)
Ecell∘=0.80V+0.76V
Ecell∘=1.56V
Now, substitute the values of n, F, and Ecell∘ into the ΔG∘ formula: Given Faraday constant F=96500 C/mol.
ΔG∘=−(2 mol)(96500 C/mol)(1.56 V)
ΔG∘=−301080 J
To express ΔG∘ in kilojoules (kJ), divide by 1000:
ΔG∘=−301.08 kJ/mol
Our calculated value is -301.08 kJ/mol. Option (A) is -305 kJ/mol. While not an exact match, it is the only option with the correct sign and is numerically the closest among the negative options.
