Question
Question: Which of the following is most reactive for S$_N$2 reaction?...
Which of the following is most reactive for SN2 reaction?
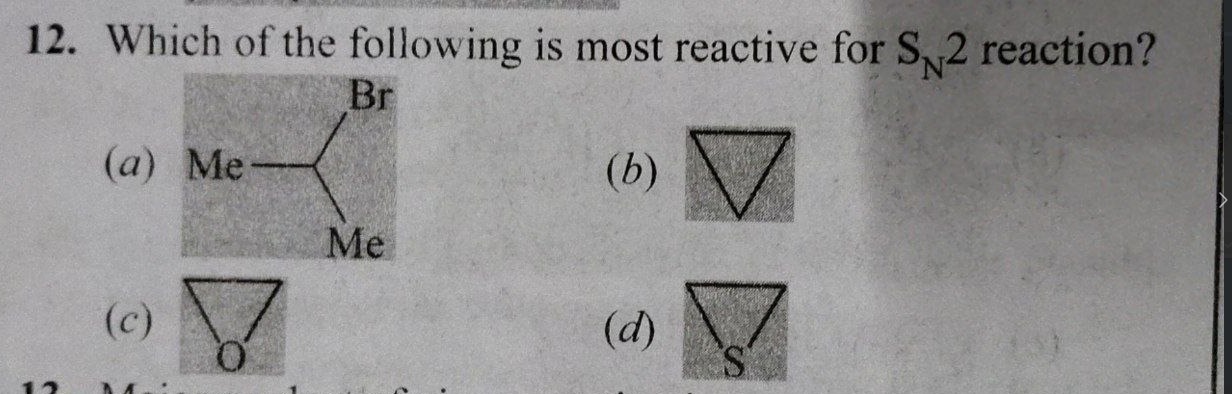
2-bromopropane
Cyclopropane
Ethylene oxide
Ethylene sulfide
Ethylene sulfide
Solution
The SN2 reaction is a bimolecular nucleophilic substitution reaction. Its rate is influenced by several factors, including steric hindrance at the reaction center, the leaving group ability, and the nature of the substrate.
- Steric Hindrance: SN2 reactions are favored by less steric hindrance. The reactivity order for alkyl halides is: methyl > primary > secondary > tertiary.
- Leaving Group: A good leaving group (e.g., halides like I−, Br−, Cl−) increases reactivity.
- Ring Strain: Cyclic compounds with strained rings, such as epoxides and thiiranes, undergo nucleophilic ring-opening reactions which are often considered SN2-like. The relief of ring strain provides a significant driving force, making these compounds very reactive.
Analyzing the options:
- 2-bromopropane: This is a secondary alkyl halide, exhibiting moderate steric hindrance.
- Cyclopropane: This is a strained ring but typically does not have a good leaving group unless substituted.
- Ethylene oxide (Oxirane): This is a three-membered cyclic ether with primary carbons, thus minimal steric hindrance. The high ring strain makes it susceptible to nucleophilic attack.
- Ethylene sulfide (Thiirane): This is a three-membered cyclic sulfide. Similar to ethylene oxide, it has primary carbons and high ring strain. Thiiranes are generally more reactive than epoxides because the C-S bond is weaker than the C-O bond, and sulfur is a larger, more polarizable atom, making it a better leaving atom.
Comparing reactivity: The relief of ring strain in thiiranes and epoxides makes them highly reactive towards nucleophiles, often exceeding the reactivity of secondary alkyl halides like 2-bromopropane. Between ethylene oxide and ethylene sulfide, the thiirane is more reactive due to the weaker C-S bond and better leaving ability of sulfur.
Therefore, ethylene sulfide is the most reactive for SN2 reaction among the given options.
