Question
Question: Volume of CO₂ obtained at STP by the complete decomposition of 9.85 g BaCO₃ is (Ba = 137)...
Volume of CO₂ obtained at STP by the complete decomposition of 9.85 g BaCO₃ is (Ba = 137)
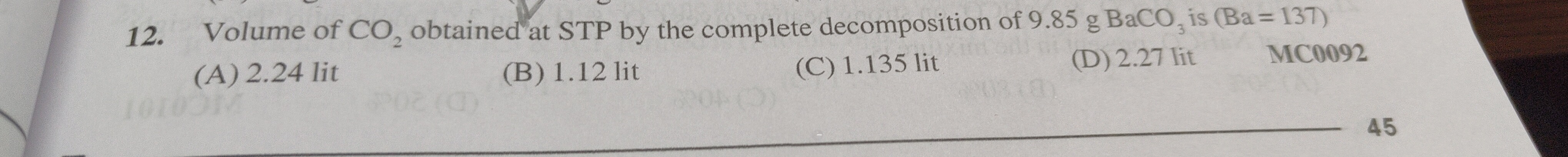
2.24 lit
1.12 lit
1.135 lit
2.27 lit
1.12 lit
Solution
Here's the breakdown of the solution:
-
Balanced Chemical Equation:
The decomposition of barium carbonate (BaCO₃) yields barium oxide (BaO) and carbon dioxide (CO₂): BaCO3(s)→BaO(s)+CO2(g) 1 mole of BaCO₃ produces 1 mole of CO₂.
-
Molar Mass of BaCO₃:
Given: Ba = 137, C = 12, O = 16 Molar mass of BaCO₃ = 137 + 12 + (3 × 16) = 197 g/mol
-
Moles of BaCO₃:
Given mass of BaCO₃ = 9.85 g Moles of BaCO₃ = Molar massMass=197g/mol9.85g=0.05mol
-
Moles of CO₂ Produced:
From the stoichiometry, 1 mole of BaCO₃ produces 1 mole of CO₂. Therefore, 0.05 moles of BaCO₃ will produce 0.05 moles of CO₂.
-
Volume of CO₂ at STP:
At STP, 1 mole of any ideal gas occupies 22.4 liters. Volume of CO₂ = Moles of CO₂ × Molar volume at STP Volume of CO₂ = 0.05mol×22.4L/mol=1.12L
