Question
Question: Identify the strongest oxidizing agent from the following species....
Identify the strongest oxidizing agent from the following species.
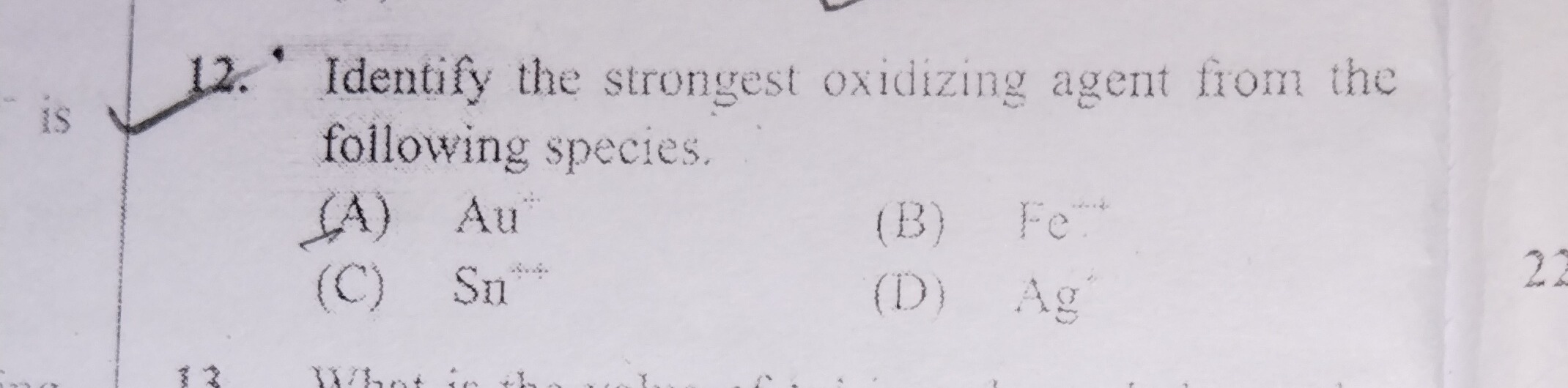
A
Au
B
Fe
C
Sn
D
Ag
Answer
Au
Explanation
Solution
An oxidizing agent is the species that is reduced, so its strength is measured by its standard reduction potential. Among the given metals, we consider the typical reduction reactions:
Au3++3e−⟶Auwith E∘≈+1.50V Ag++e−⟶Agwith E∘≈+0.80V Fe2++2e−⟶Fewith E∘≈−0.44V Sn2++2e−⟶Snwith E∘≈−0.14VSince the most positive standard reduction potential corresponds to the strongest tendency to gain electrons (i.e. the strongest oxidizing agent), gold (Au) is the strongest oxidizing agent among the given options.
Core Explanation (Minimal):
- Oxidizing agent strength is determined by the standard reduction potential.
- Au (via Au³⁺/Au couple) has the highest reduction potential (+1.50 V) compared to Ag, Fe, and Sn.
- Hence, Au is the strongest oxidizing agent.
