Question
Question: For the two reactions I: A → B; II: C → D following graph is obtained. Which of the following is tr...
For the two reactions I: A → B; II: C → D following graph is obtained.
Which of the following is true:
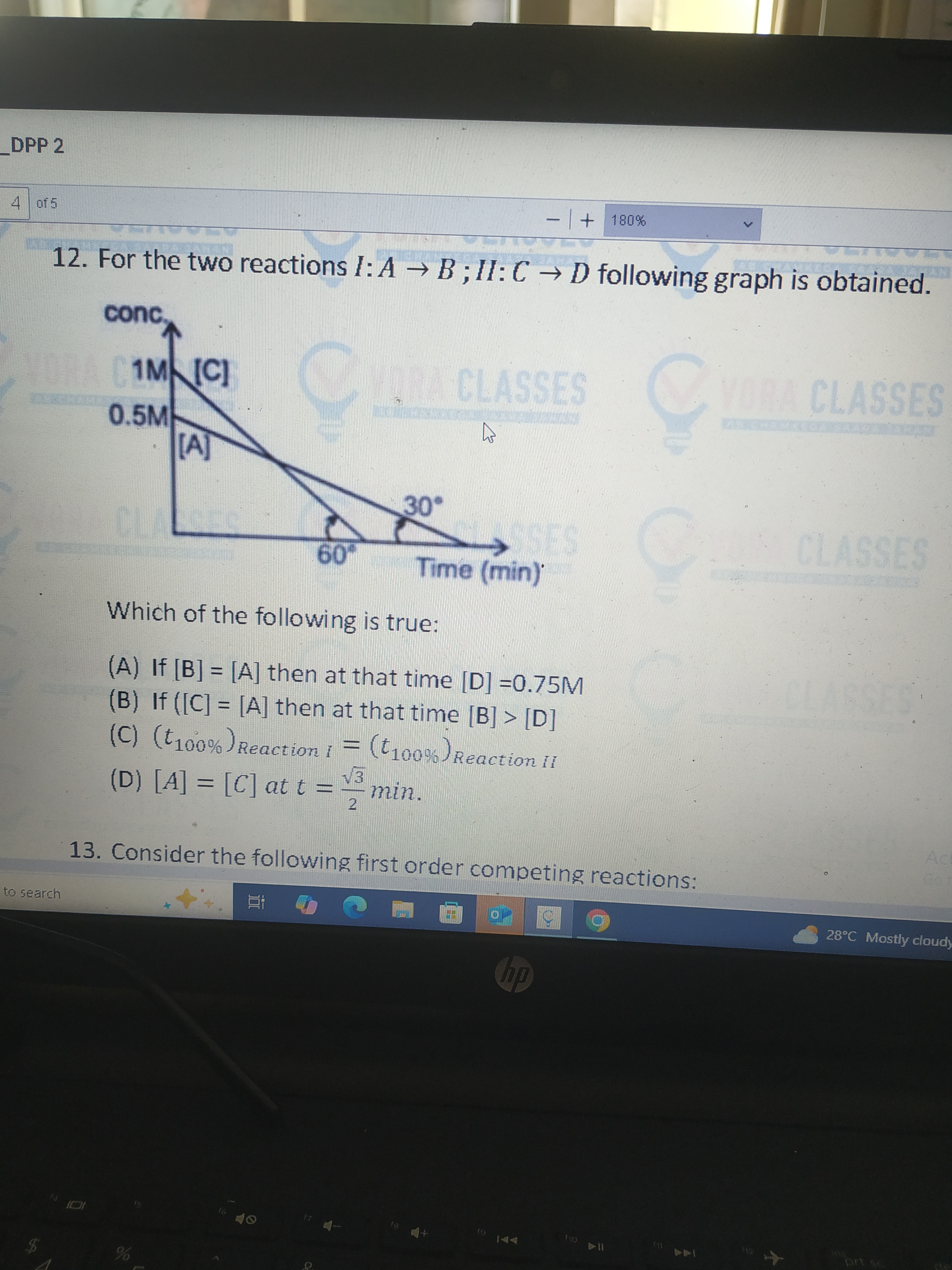
If [B] = [A] then at that time [D] =0.75M
If ([C] = [A] then at that time [B] > [D]
(t100%)ReactionI = (t100%)ReactionII
[A] = [C] at t = 23 min.
If [B] = [A] then at that time [D] = 0.75M
Solution
The problem describes two zero-order reactions, I: A → B and II: C → D, and provides a graph of reactant concentration versus time.
1. Determine Initial Concentrations and Rate Constants: From the graph:
- Initial concentration of A, [A]0=0.5 M.
- Initial concentration of C, [C]0=1 M.
For a zero-order reaction, the rate law is: Rate =k The integrated rate law is: [R]t=[R]0−kt The slope of the concentration vs. time graph is −k.
The angles shown in the graph are 60° for reaction I (A) and 30° for reaction II (C).
Let's assume the angles given (60° and 30°) are with respect to the concentration (vertical) axis. This means the angles with the time (horizontal) axis would be 90∘−60∘=30∘ for reaction I, and 90∘−30∘=60∘ for reaction II. With this interpretation:
- For reaction I: Angle with time axis = 30°. Slope = −tan(30∘)=−1/3. So, kI=1/3 M/min.
- For reaction II: Angle with time axis = 60°. Slope = −tan(60∘)=−3. So, kII=3 M/min.
2. Evaluate Each Option:
A) If [B] = [A] then at that time [D] = 0.75M For reaction I: A → B, [B]t=[A]0−[A]t. Given [B]t=[A]t: [A]t=[A]0−[A]t⟹2[A]t=[A]0⟹[A]t=[A]0/2. So, [A]t=0.5/2=0.25 M. Now find the time t when [A]t=0.25 M: [A]t=[A]0−kIt 0.25=0.5−(1/3)t (1/3)t=0.5−0.25=0.25 t=0.253=43 min.
Now find [D] at this time t=43 min for reaction II: C → D. [D]t=[C]0−[C]t. Since C → D, [D] formed = [C] reacted. [D]t=kIIt (since [D]0=0) [D]t=3×43=43=0.75 M. This matches the statement in option (A). So, option (A) is true.
Final Answer: The final answer is A
Subject: Chemistry Chapter: Chemical Kinetics Topic: Zero Order Reactions, Integrated Rate Law, Graphical Representation of Reaction Kinetics
