Question
Question: Calculate n-f (for only Reacent) $KMnO_4 \rightarrow MnO_4^{2-}$ $FeO Fe_{0.9}O \rightarrow FeO$ ...
Calculate n-f (for only Reacent)
KMnO4→MnO42−
FeOFe0.9O→FeO
CH3P→H3PO4
FeS2→SO2
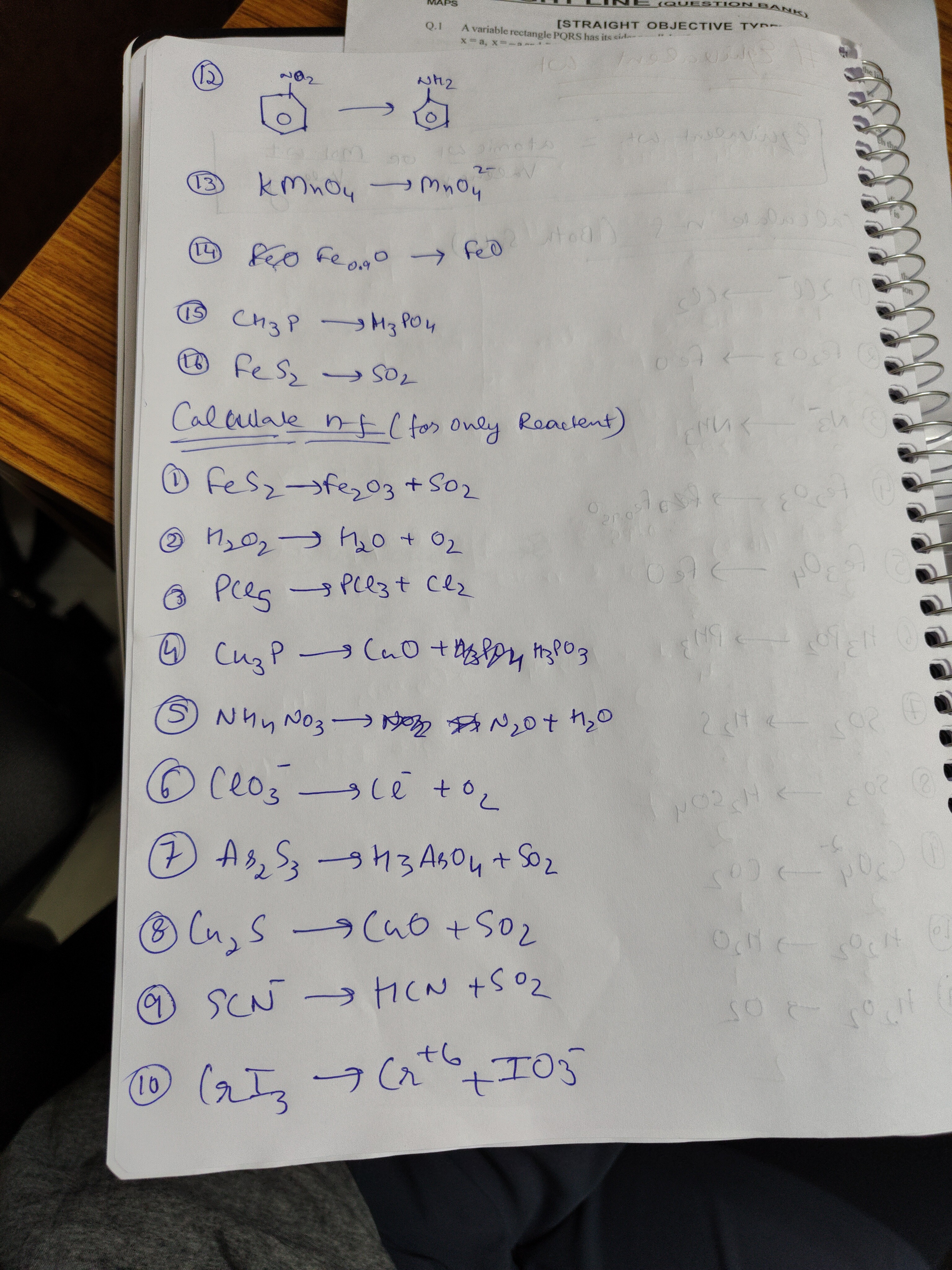
Answer
The n-factors for the reactants are:
- (12) C₆H₅NO₂: 6
- (13) KMnO₄: 1
- (14) Fe₀.₉O: 0.2
- (15) CH₃P: 6
- (16) FeS₂: 11
- (1) FeS₂: 11
- (2) H₂O₂: 2
- (3) PCl₅: 2
- (4) Cu₃P: 11
- (5) NH₄NO₃: 8
- (6) ClO₃⁻: 6
- (7) As₂S₃: 22
- (8) Cu₂S: 8
- (9) SCN⁻: 8
- (10) CrI₃: 21
Explanation
Solution
The n-factor (or valency factor) for a reactant in a redox reaction is calculated as the total change in oxidation state of all atoms in one molecule (or formula unit) of the reactant that undergo oxidation or reduction.
- Assign oxidation states: Determine the oxidation state of each relevant atom in the reactant and its corresponding product(s).
- Calculate change per atom: Find the absolute difference between the initial and final oxidation states for each atom that changes.
- Multiply by stoichiometry: Multiply the change per atom by the number of such atoms present in one formula unit of the reactant.
- Sum changes: If multiple different atoms within the same reactant formula unit undergo oxidation or reduction (or both, as in disproportionation or mixed redox compounds), sum up the absolute values of these total changes.
