Question
Question: 12. ...
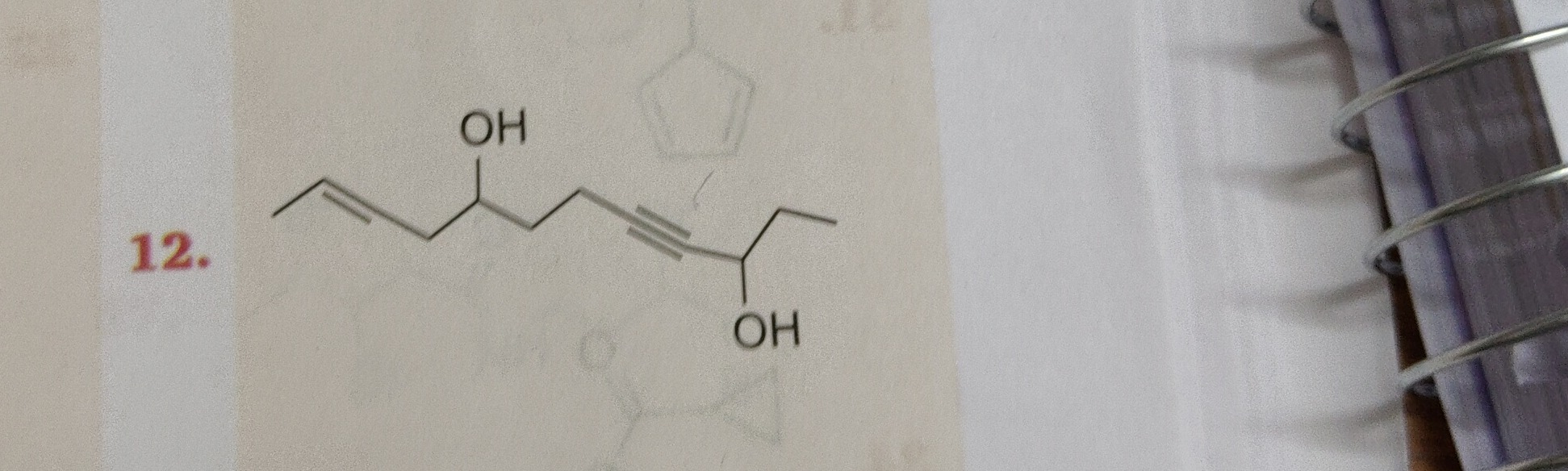
Deca-9-en-4-yne-3,7-diol
Solution
To name the given organic compound using IUPAC nomenclature, follow these steps:
-
Identify the longest continuous carbon chain: The molecule contains 10 carbon atoms. Therefore, the parent chain is "dec-".
OH OH | | CH2=CH-CH2-CH-CH2-C≡C-CH-CH2-CH3 -
Identify and prioritize functional groups:
- Two hydroxyl groups (-OH) are present, which are the principal functional groups (alcohols).
- One double bond (alkene).
- One triple bond (alkyne).
Alcohols have higher priority than alkenes and alkynes. Therefore, the carbon chain must be numbered to give the lowest possible locants to the hydroxyl groups.
-
Number the carbon chain:
-
Option 1: Numbering from left to right (L-R)
1 2 3 4(OH) 5 6 7 8(OH) 9 10 CH2=CH-CH2-CH-CH2-C≡C-CH-CH2-CH3- Hydroxyl groups at C4 and C8. (Locants: 4, 8)
- Double bond at C1.
- Triple bond at C6.
-
Option 2: Numbering from right to left (R-L)
Let's rewrite the molecule from right to left for clarity:
CH3-CH2-CH(OH)-C≡C-CH2-CH(OH)-CH2-CH=CH2 1 2 3(OH) 4 5 6 7(OH) 8 9 10- Hydroxyl groups at C3 and C7. (Locants: 3, 7)
- Triple bond at C4.
- Double bond at C9.
Comparing the locants for the principal functional groups (hydroxyl groups):
- L-R: (4, 8)
- R-L: (3, 7)
The set (3, 7) is lower than (4, 8). Therefore, numbering from right to left is correct.
-
-
Construct the IUPAC name:
-
Parent chain: Deca- (10 carbons). The 'a' is retained because there are multiple multiple bonds (ene and yne) and a polyfunctional suffix (-diol).
-
Principal functional group suffix: -3,7-diol (for -OH groups at C3 and C7).
-
Multiple bonds:
- Double bond at C9: 9-ene
- Triple bond at C4: 4-yne
-
When both double and triple bonds are present, the 'ene' suffix comes before the 'yne' suffix alphabetically. The 'e' from 'ene' is dropped when followed by 'yne'. The 'e' from 'yne' is retained when followed by a suffix starting with a consonant (like 'd' in diol).
Combining these parts:
Deca-9-en-4-yne-3,7-diol -
The final IUPAC name is Deca-9-en-4-yne-3,7-diol.
The structure can be represented as:
C(C=C)CC(O)CC#CC(O)CC
Explanation of the Solution:
- Identify Parent Chain: The longest carbon chain is 10 carbons, so "dec-".
- Prioritize Functional Groups: Alcohols (-OH) have higher priority than alkenes and alkynes.
- Numbering: Number the chain to give the lowest locants to the alcohol groups. Numbering from right to left gives -OH at C3 and C7 (3,7-diol), which is lower than C4 and C8 if numbered from left to right.
- Locate Multiple Bonds: Based on R-L numbering, the triple bond is at C4 (4-yne) and the double bond is at C9 (9-ene).
- Assemble Name: The name follows the format: parent-chain-ene-yne-diol. The 'a' in 'deca' is retained due to multiple multiple bonds. 'ene' comes before 'yne'. The 'e' of 'ene' is dropped before 'yne'. The 'e' of 'yne' is retained before 'diol' (as 'd' is a consonant).
