Question
Question: A compound of vanadium has a magnetic moment ($\mu$) of 1.73 BM. If the vanadium ion in the compound...
A compound of vanadium has a magnetic moment (μ) of 1.73 BM. If the vanadium ion in the compound is present as Vx+, then, the value of x is?
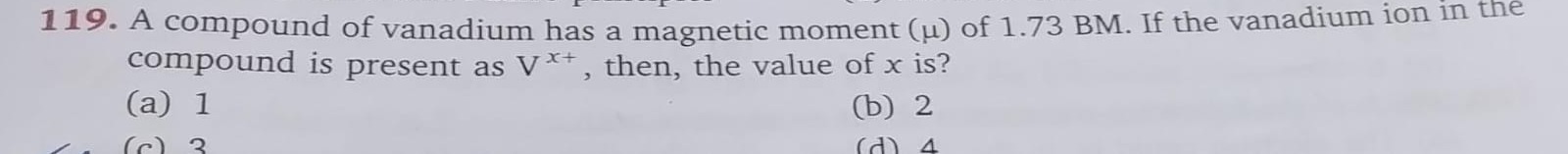
1
2
3
4
4
Solution
The magnetic moment (μ) of a transition metal ion is given by the formula μ=n(n+2) BM, where n is the number of unpaired electrons. Given μ=1.73 BM: 1.73=n(n+2) (1.73)2≈n(n+2) 2.9929≈n(n+2) By inspection, if n=1, n(n+2)=1(1+2)=3. Thus, the number of unpaired electrons is n=1.
Vanadium (V) has atomic number 23, with electronic configuration [Ar]4s23d3. We need to find the charge x of the vanadium ion (Vx+) that results in 1 unpaired electron. Electrons are removed from the outermost shell (4s) first, followed by the 3d subshell.
- Neutral V: [Ar]4s23d3 (3 unpaired electrons in 3d)
- V1+: [Ar]4s13d3 (4 unpaired electrons)
- V2+: [Ar]3d3 (3 unpaired electrons)
- V3+: [Ar]3d2 (2 unpaired electrons)
- V4+: [Ar]3d1 (1 unpaired electron)
- V5+: [Ar]3d0 (0 unpaired electrons)
The configuration [Ar]3d1 yields n=1 unpaired electron, which matches the calculated value. This configuration corresponds to the V4+ ion, meaning x=4.
