Question
Question: Identify an aromatic, mixed, 3º amine among the following compounds....
Identify an aromatic, mixed, 3º amine among the following compounds.
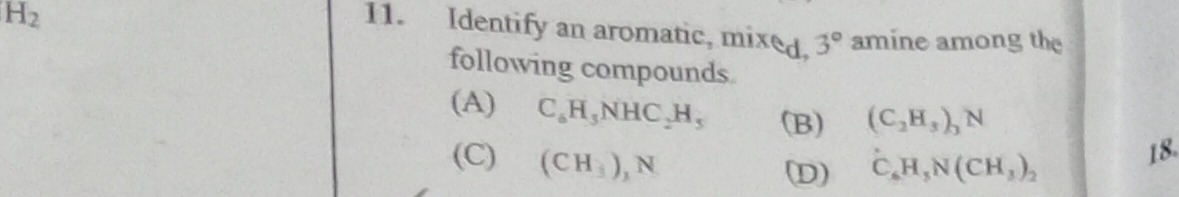
A
C6H5NHC2H5
B
(C2H5)3N
C
(CH3)3N
D
C6H5N(CH3)2
Answer
Option (D)
Explanation
Solution
For a compound to be a tertiary (3º) amine, the nitrogen atom must be bonded to three carbon groups. To be aromatic, at least one of these groups must be an aromatic ring. Examining the options:
- (A) C6H5NHC2H5: Here, the nitrogen is bonded to a phenyl group and an ethyl group. This is a secondary amine.
- (B) (C2H5)3N: This is a tertiary amine but all three groups are ethyl groups (no aromatic ring).
- (C) (CH3)3N: This is a tertiary amine with three methyl groups (again, no aromatic ring).
- (D) C6H5N(CH3)2: Here, the nitrogen atom is bonded to one phenyl group (aromatic) and two methyl groups, making it a mixed tertiary amine.
Thus, the aromatic, mixed, 3º amine is option (D).
