Question
Question: 100 ml of a sample of hard water requires 20 ml of 0.03 N H\textsubscript{2}SO\textsubscript{4} for ...
100 ml of a sample of hard water requires 20 ml of 0.03 N H\textsubscript{2}SO\textsubscript{4} for complete reaction. The hardness of water (density = 1 gm/ml) is:
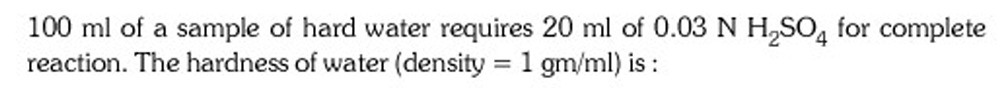
300 mg/L
Solution
Solution:
-
Calculation of equivalents consumed by acid:
Volume of H\textsubscript{2}SO\textsubscript{4} = 20 mL = 0.02 L
Normality = 0.03 N
Equivalents in 100 mL water = 0.02 × 0.03 = 0.0006 eq
-
Extrapolate to 1 L of water:
Since 100 mL has 0.0006 eq, 1 L has
- 0006 × 10 = 0.006 eq
-
Convert equivalents to mg CaCO\textsubscript{3} (using CaCO\textsubscript{3} equivalent weight = 50 mg/meq):
Hardness = 0.006 eq/L × 1000 mg/eq (because 1 eq of CaCO\textsubscript{3} = 50 g = 50,000 mg divided by 1000 if 50 mg per meq)
Alternatively, in meq: 0.006 eq = 6 meq.
Hardness (mg CaCO\textsubscript{3}) = 6 meq/L × 50 mg/meq = 300 mg/L
Explanation:
Calculate equivalents of acid used in 100 mL sample, scale to 1 L (multiply by 10) to get 6 meq/L, then multiply by 50 (mg/ meq conversion factor) to determine hardness as 300 mg/L CaCO\textsubscript{3}.
